T lymphocytes are an important element of the adaptive immune response. While normally quiescent, T cells can replicate rapidly to build an army of antigen-specific cells in only a few days following an immune challenge. Many of the cells created are short-lived and die within the first 2 weeks, whereas other cells return to a quiescent long-lived state and serve as memory cells capable of faster, more effective secondary responses. The division history of cells that transit between the naïve, effector and memory states has been mysterious and controversialCitation1,2 but is recently illuminated by experiments that take advantage of the Fucci cell cycle reporter mouse.Citation3 Cells from this mouse exhibit ‘red’ fluorescence during G1 (due to fusion of mKusabira-Orange 2 to hCdt1(30/120)) and green fluorescence in S/G2/M (fusion of mAzami-Green to hGeminin(1/110)). A critical additional feature is that the intensity of red fluorescence increases with the time cells spend in the G1 phase (or G0) phase.Citation4-6 Cells that are non-cycling, or quiescent become bright red, exemplified by the pool of resting T cells in unstimulated animals (see ).Citation4-6
Figure 1. Tracking lymphocyte dynamics with Fucci reporters. (A) Lymphocytes are typically ‘resting’ allowing the G1 Fucci(red) fluorescence indicator to accumulate to high levels. If stimulated, cells initiate a series of division rounds where the time spent in G1 is short and red fluorescence weak. Cells return to a quiescent resting state and dilution of dye CTV (bar at the top) allows discrimination of resting memory from the naïve cells. (B) The number of division rounds T cells undergo varies depending on the strength and combination of signals received. (C) Direct time-lapse imaging reveals additional features of cell cycle control by lymphocytes.
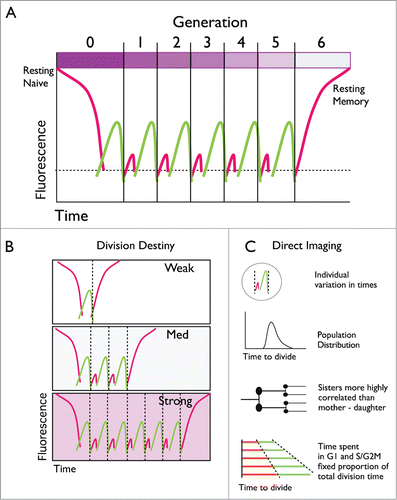
To study the dynamics of T cell cycle transitions during an infection, Fucci mice were crossed to the OT-I T cell receptor transgenic mouse in 2 recent studies.Citation5,6 OT-I T cells are reactive to an ovalbumin (OVA) peptide. By transferring a small number of these cells to wild-type host mice infected with an influenza virus that expresses the relevant OVA peptide dynamic cell cycle changes can be followed. Transferred T cells were also labeled with the division tracking dye, cell trace violet (CTV). Influenza infection stimulated the T cells to undergo a series of division cycles where little time is spent in the G1 phase ensuring the red fluorescence is weak, and, in many cells, not detectable.Citation5,6 Surprisingly T cells with a memory phenotype began to emerge at the height of the T cell response (7 days following infection), and were also high in red fluorescence indicating their return to a slow division rate.Citation5 These cells had also diluted out the CTV dye indicating multiple divisions. As OT-I cells at earlier times were low in red fluorescence and displayed an effector cell phenotype (i.e. intermediate to low CD62L), the results indicated that the new quiescent memory cells had emerged from a rapidly proliferating precursorCitation5 (). Mathematical modeling estimated the number of division cycles T cells underwent before returning to a quiescent state in this system as ranging from 5–15.Citation6
This pattern of intense proliferation followed by exit to a slow, or non-dividing quiescent state was also observed for cells cultured in vitro. T cells sorted from early generations and returned to culture continued to divide rapidly in vitro. In contrast, cells from later generations often slowed down markedly, or did not divide at all, apparently re-emerging as a quiescent cell.Citation5 By stripping away all costimulatory and cytokine signals Marchingo et al. identified regulators of the number of division cycles cells undergo before ceasing to divide.Citation6 These signals could be added together and summed with a simple linear addition rule. Furthermore, the division program (termed the cell's division destiny) could be imprinted prior to the first cell division and carried through multiple generations. Together both in vivo and in vitro studies imply that T cells may divide and return to a resting state in a cell intrinsic, autonomous manner. A similar pattern of response has also been seen for the other arm of the adaptive immune response, the antibody secreting B lymphocyte, suggesting a general principle for lymphocyte cell cycle control has been uncovered.Citation7 These studies do not solve the problem of where, or how, a rich variety of alternative cell fates, including memory cells, arise during the initial burst of T or B cell proliferation. Direct cell imaging is helping answer this question.
As found for all cell types there is considerable variation in division times of cycling T and B lymphocytes.Citation4,5,7 Despite this variation, correlation between times for 2 sibling cells is extremely high. For T cells measurements are from 0.84 to 0.98 (Spearman's rho)Citation4,5 and for B cells 0.86 to 0.94.Citation4,7 There was also a high concordance for sisters to share the slow division time at late generations.Citation5 The similar fates were not due to the shared local conditions: Kinjyo et al. followed individually marked red and green fluorescent T cells in the same small microwell. Correlations of the times to divide of siblings remained extremely high, and much higher than local neighbors. Thus, 2 sibling cells, as molecular clones, have similar fates. They also share a fixed proportion of time in each cell cycle phase.Citation4 The stochastic element in the choice of division time appears to come earlier as mother-daughter division times show significantly weaker correlation (in the order 0.32–0.65 for both T and B cells), indicating that the scrambling of division times is carried out, in part, by the mother before passing on similar times to both daughters.
There is much yet to learn about how lymphocytes select their individual division paths but these recent insights illustrate how general principles from single cell studies can be used in combination with cell cycle reporters to illuminate our understanding of the complex dynamic immune system.
References
- Gerlach C, et al. Science 2013; 340:635-9; PMID:23493421; http://dx.doi.org/10.1126/science.1235487
- Buchholz V R, et al. Science 2013; 340:630-5; PMID:23493420; http://dx.doi.org/10.1126/science.1235454
- Sakaue-Sawano A, et al. Cell 2008; 132:487-98; PMID:18267078; http://dx.doi.org/10.1016/j.cell.2007.12.033
- Dowling MR, et al. Proc Natl Acad Sci U S A 2014; 111:6377-82; PMID:24733943; http://dx.doi.org/10.1073/pnas.1322420111
- Kinjyo I, et al. Nature Commun 2015; 6:6301; PMID:25709008; http://dx.doi.org/10.1038/ncomms7301
- Marchingo JM, et al. Science 2014; 346:1123-7; PMID:25430770; http://dx.doi.org/10.1126/science.1260044
- Hawkins ED, et al. Proc Natl Acad Sci U S A 2009; 106:13457-62; PMID:19633185; http://dx.doi.org/0905629106 [pii]10.1073/pnas.0905629106
