Abstract
Recently, we reported that saturated and unsaturated fatty acids trigger autophagy through distinct signal transduction pathways. Saturated fatty acids like palmitate (PA) induce autophagic responses that rely on phosphatidylinositol 3-kinase, catalytic subunit type 3 (PIK3C3, best known as VPS34) and beclin 1 (BECN1). Conversely, unsaturated fatty acids like oleate (OL) promote non-canonical, PIK3C3- and BECN1-independent autophagy. Here, we explored the metabolic effects of autophagy-inducing doses of PA and OL in mice. Mass spectrometry coupled to principal component analysis revealed that PA and OL induce well distinguishable changes in circulating metabolites as well as in the metabolic profile of the liver, heart, and skeletal muscle. Importantly, PA (but not OL) causes the depletion of multiple autophagy-inhibitory amino acids in the liver. Conversely, OL (but not PA) increased the hepatic levels of nicotinamide adenine dinucleotide (NAD), an obligate co-factor for autophagy-stimulatory enzymes of the sirtuin family. Moreover, PA (but not OL) raised the concentrations of acyl-carnitines in the heart, a phenomenon that perhaps is linked to its cardiotoxicity. PA also depleted the liver from spermine and spermidine, 2 polyamines have been ascribed with lifespan-extending activity. The metabolic changes imposed by unsaturated and saturated fatty acids may contribute to their health-promoting and health-deteriorating effects, respectively.
Introduction
Saturated and unsaturated fatty acids have a different impact on human health. The most abundant fatty acid in human tissues, i.e., palmitate (PA; C16:0), is contained in potentially harmful vegetal oils like palm oil. In contrast, the most abundant unsaturated fatty acid, i.e., oleic acid or oleate (OL; C18:1), is a major component of olive oil, which is commonly associated with the health-promoting effects of the Mediterranean diet.Citation1-4
Both saturated and unsaturated fatty acids are known to stimulate autophagy, an adaptive response to stress that is common to all eukaryotes and consists in the sequestration of cytoplasmic entities (including lipid vesicles) within double-membraned vesicles (known as autophagosomes) followed by their lysosomal degradation.Citation5-8 However, we recently discovered that saturated and unsaturated fatty acids trigger autophagy via radically different molecular mechanisms.Citation9,10 Saturated fatty acids including PA stimulate indeed a classical autophagic response that relies on the activation of phosphatidylinositol 3-kinase, catalytic subunit type 3 (PIK3C3, best known as VPS34) within a multiprotein complex that contains beclin 1 (BECN1).Citation11,12 PIK3C3 catalyzes the conversion of phosphatidylinositol into phosphatidylinositol 3-phosphate (PtdIns3P), a lipid that is required for the initiation of autophagy in response to most stimuli including nutrient and growth factor deprivation.Citation13 In sharp contrast, unsaturated fatty acids including OL fail to cause the activation of PIK3C3 and boost autophagic flux in the absence of BECN1 or PIK3C3. Such a non-canonical, BECN1- and PIK3C3-independent autophagic response appears to be evolutionarily conserved, and hence physiologically relevant. Indeed, all 4 species investigated in this respect (i.e., human cancer cells, the yeast Saccharomyces cerevisiae, the nematode Caenorhabditis elegans, and laboratory mice) mounted normal autophagic responses to OL even in the absence of BECN1 or its orthologs.Citation9
Although our results clearly indicate that PA and OL trigger autophagy through distinct signaling pathways, the underlying molecular mechanisms remain obscure. Driven by this unknown, we treated mice with doses of PA and OL that induce a quantitatively similar (though mechanistically distinct) autophagic response. We then determined the biochemical and metabolic consequences of these treatments by mass spectrometry. Here, we report that PA and OL induce rather distinct changes in circulating metabolites as well as in the intracellular metabolic profiles of the liver, heart, and skeletal muscle. These alterations may explain, at least in part, the differential impact of saturated and unsaturated fatty acids on human health, as well as the distinct mechanisms through which they trigger autophagy.
Results and Discussion
Metabolic changes induced by PA and OL in mouse tissues
Standard laboratory mice were injected intraperitoneally with 100 mg/Kg PA (5 mice), 100 mg/Kg OL (5 mice) or an equivalent volume of vehicle (5 mice), and euthanatized 6 hours later in order to recover the serum and 3 organs, i.e., the liver, heart and gastrocnemius (as a representative skeletal muscle). Samples were processed according to standard procedures for metabolite extraction and subjected to chromatography and mass spectrometry, in both open profiling and targeted modes.Citation14 We selected this approach as it would enable us to monitor well-known metabolites as well as compounds not so frequently assessed in biological studies (but perhaps important for our experimental setting). This procedure yielded 1,268 features (i.e., reproducible retention times and masses) for the liver, 368 of which were bona fide, precisely identified or putatively assigned metabolites; 1,110 (293) features for the heart; 873 (262) features for the skeletal muscle; and 1,050 (197) features for the serum. In total, our approach yielded 2,598 features among which 304 were putatively assigned and non-redundant metabolites, and 211 were bona fide, precisely identified metabolites (, Fig. S1 and Table S1). After data normalization, linear discriminant analyses (LDAs) highlighted that the metabolic profiles of mice receiving vehicle, PA or OL differ. These findings were obtained when the totality of features (including identified, putative and unknown metabolites) were subjected to LDA (), as well as when the LDA was restricted on identified and putative metabolites (). These data demonstrate that PA and OL induce a series of well distinguishable metabolic changes.
Figure 1. Global metabolic setup of the samples analyzed in this study. Mice were injected intraperitoneally with 100 mg/Kg palmitate (PA, 5 mice), 100 mg/Kg oleate (OL, 5 mice), or an equivalent volume of vehicle (Co, 5 mice), euthanatized 6 hrs later and processed for the analysis of small metabolites in the liver, heart, skeletal muscle and serum. Heat maps depict metabolites that were detected with reproducible retention times, mass to charge ratios and intensities in all 4 data sets (A), in tissues only (B), or in any other dataset combination (C). Features that were not detected or had a signal intensity < blank are depicted in gray. Detected features that failed quality controls are depictured in blue. Abbreviations and additional data are provided in Table S1.
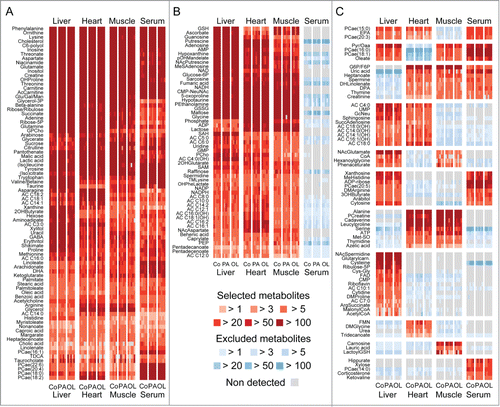
Figure 2. Linear discriminant analysis and statistical assessment of the metabolic changes caused by palmitate or oleate in vivo. A–D. All features (A,C) or identified metabolites (B, D) were subjected to linear discriminant analysis for treatment-related variation (A, B) or studied with respect to p value frequency (C, D). In panels A and B, each dot represents an individual mouse. In panels C and D, the dashed line depicts the density of p values expected if all metabolites were not altered by PA or OL. CCC, canonical correlation coefficient (significant if > 0.8); Co, vehicle; PA, palmitate; OL, oleate.
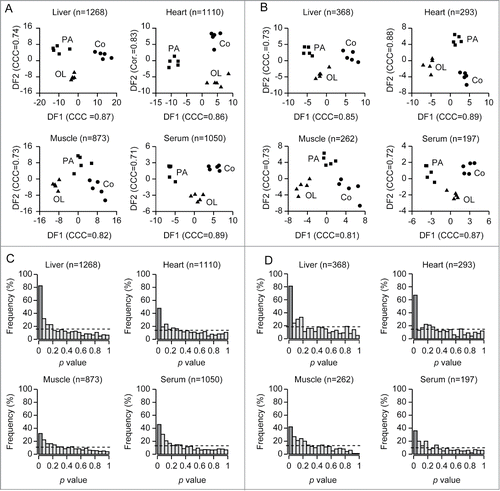
The number of significant (p < 0.05, moderated F-test) metabolic alterations induced by either PA or OL was highest in the liver, followed by the heart, serum and skeletal muscle, both for the totality of the features () and putative plus identified metabolites (). In the liver, the levels of 81 among 368 identified metabolites (21%) changed significantly in response to PA or OL (), while that of 67 among 293 cardiac metabolites (23%) did so (). These proportions were smaller for the serum, in which the abundance of only 36 among 197 metabolites (18%) changed upon the administration of PA or OL (), and for the skeletal muscle, in which such a significant quantitative shift involved 42 among 262 metabolites (16%) (). Thus, the short-term effects of fatty acids administered intraperitoneally are most evident in the liver, possibly as a direct consequence of portal circulation. There are also subtle inter-organ differences in the short-term metabolic effects of fatty acids, which will be described in the following section.
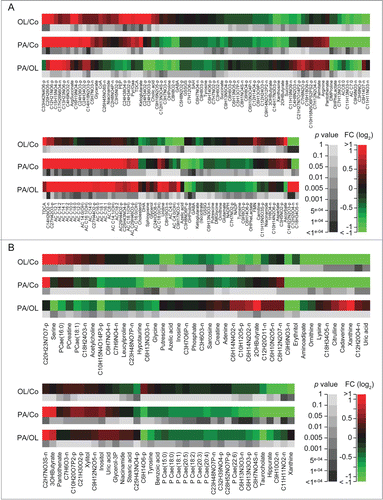
Figure 3. Effects of fatty acids on identified metabolites in vivo. A–D. Heat maps depict the effects of intraperitoneal palmitate (PA) or oleate (OL), as compared to each other (PA/OL) or to vehicle (PA/Co and OL/Co), on metabolites identified in the liver (A), heart (B), serum (C) and skeletal muscle (D), and the corresponding p values (moderated F-test). Only comparisons associated with overall p values < 0.05 were included. Data are presented as fold changes (FCs) in log2 scale.
PA and OL alter the abundance of key regulators of aging and autophagy
Amino acids are among the most efficient endogenous repressors of autophagy.Citation15-17 PA significantly reduced the hepatic levels of threonine, proline, tyrosine, glycine, valine and ornithine (a non-proteogenic amino acid that is essential for polyamine biosynthesis), but increased that of arginine and histidine (). Conversely, OL (but not PA) increased the hepatic levels of aspartic acid (). PA also caused a hepatic depletion of spermine and spermidine (2 polyamines that stimulates autophagy)Citation18-20 (). This was accompanied by the accumulation of N-acetylspermidine (), which constitutes the form of spermidine that can be released from cells,Citation21 potentially explaining the drop in the abundance of spermidine, spermine and ornithine. Concordant with its effects on proline, PA (but not OL) also reduced the hepatic concentration of hydroxyproline (), possibly reflecting enhanced collagen biosynthesis. PA (but not OL) caused a hepatic depletion of the glutamic acid derivative γ-aminobutyric acid (GABA), as it provoked the accumulation of N-acetylglutamine, creatine and phosphocreatine (). Moreover, there were subtle differences in the effects of PA and OL on the tricarboxylic acid (TCA) cycle, which is intimately connected to autophagy via acetyl-coenzyme A (acetyl-CoA).Citation16,22-25 Thus, PA (but not OL) depleted hepatocytes from succinate, but favored the accumulation of isocitrate and citrate. Moreover, PA (but not OL) caused a reduction in the hepatic levels of cholic acid and of the acetyl-CoA derivative malonyl-CoA, which plays a key role in lipid biosynthesis ( and Fig. S2). Importantly, OL (but not PA) stimulated the hepatic accumulation of niacinamide and its products nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP), as well as that of the CoA precursor panthotenic acid ().
Figure 4. Metabolic effects of fatty acids in the liver. A–C. Histograms depict the effects of intraperitoneal palmitate (PA) or oleate (OL), as compared to each other (PA/OL) or to vehicle (PA/Co and OL/Co), on the hepatic levels of amino acids (A), amino acid-related molecules (B) and other metabolites (C). Data are presented as fold changes (FCs) and associated 95% confidence intervals, in log2 scale. * p < 0.05, ** p < 0.01, *** p < 0.001 (moderated F-test).
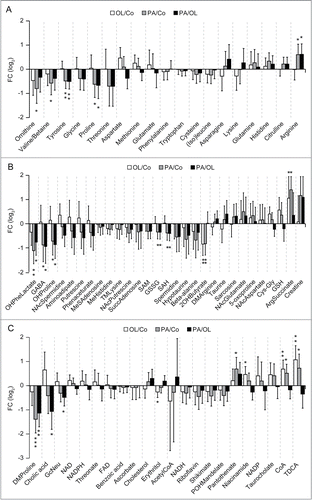
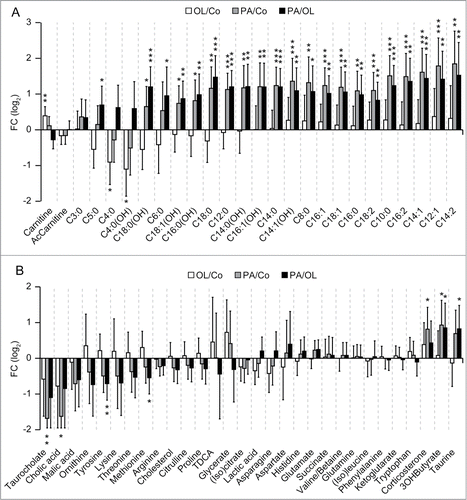
In the heart, PA (but not OL) depleted tyrosine, glutamic acid, lysine, methionine, phenylalanine, as well as ornithine (Fig. S3). Importantly, PA (but not OL) increased the concentration of multiple acyl-carnitines in the myocardium (), as well as the cardiac levels of niacinamide and flavine mononucleotide (FMN) (Fig. S3). Moreover, PA (but not OL) decreased the circulating amounts of spermine, tyrosine, threonine, taurodeoxycholic acid and taurocholic acid, as it increased those of 3-hydroxybutyric acid, a ketone body that is produced as the result of lipolysis ().Citation26 Conversely, OL increased the serum concentration of taurine (), pointing to other subtle differences in systemic metabolic alterations induced by PA and OL.
Figure 5. Metabolic effects of fatty acids in the heart and serum. (A, B) Histograms depict the effects of intraperitoneal palmitate (PA) or oleate (OL), as compared to each other (PA/OL) or to vehicle (PA/Co and OL/Co) on the cardiac levels of acyl-carnitines (A), and on the circulating levels of other metabolites (B). Data are presented as fold changes (FCs) and associated 95% confidence intervals, in log2 scale. * p < 0.05, ** p < 0.01, *** p < 0.001 (moderated F-test).
Concluding remarks
Although PA and OL are chemically related, our mass spectrometry-based studies indicate that they have rather dissimilar short-term metabolic effects in vivo. Several metabolic perturbations induced by PA (but not by OL) may explain how this fatty acid induces autophagy. In particular, the administration of PA (but not that of OL) caused the depletion of endogenous inhibitors of autophagy like amino acids, especially in the liver. In addition, PA (but not OL) increased the cardiac concentration of several acyl-carnitines. This is in line with previous data indicating that the knockdown of carnitine palmitoyltransferase 1A (CPT1A), a mitochondrial enzyme that produces acyl-carnitines, inhibits PA-induced autophagy, but not autophagic responses driven by OL.Citation9 Moreover, the accumulation of acyl-carnitines in the heart of mice receiving PA may be connected to the well-known cardiotoxicity of saturated fatty acids.
Importantly, PA (but not OL) caused the hepatic depletion of 2 pro-autophagic polyamines, spermine and spermidine. This was accompanied by a reduction in the hepatic levels of ornithine (a polyamine precursor) as well as by the accumulation of acetyl-spermidine (a product of spermidine catabolism). Thus, PA appears to boost the consumption of catabolism of polyamines. Interestingly, the tissue levels of spermine and spermidine are known to decrease with aging,Citation27 and their supplementation extends healthy lifespan in multiple model organisms including yeast, nematodes, and mice.Citation19,20,28-32 Conversely, OL (but not PA) increased the hepatic concentrations of NAD, its precursor niacinamide, and its derivative NADP. NAD is a major activator of sirtuins, which are deacetylases that stimulate autophagy and have anti-aging properties.Citation33,34
Altogether, these results suggest that PA may favor and OL counteract the activation of processes linked to aging. Given the tight link between aging and the metabolic syndrome (which is also associated with the an excessive intake of saturated fatty acids),Citation35,36 these findings may explain (at least in part) the diametrically opposed effects on human health of saturated and unsaturated fatty acids.
Materials and Methods
Mouse experiments
Six-weeks old female C57BL/6 mice (Janvier Labs, Le Genest Saint Isle, France) were bred and maintained in compliance with the FELASA guidelines, the European Community regulations for animal experiments (2010/63UE), and the Local Ethics Committee for Animal Welfare (CE n. Twenty-six: 2012–65, 2012–67; Val de Marne, France). Mice were housed in a temperature-controlled environment with 12 h light/dark cycles, and received food and water ad libitum. Animals were intraperitoneally administered with a single dose of 100 mg/Kg PA or 100 mg/Kg oleate in 10% BSA solution (w:v in PBS), or with an equivalent volume of vehicle, and were euthanized 6 hours later. Immediately thereafter, serum and tissues were recovered, snap-frozen in liquid nitrogen and kept at −80°C until metabolite extraction.
Sample preparation
Snap-frozen tissue fragments of 60–80 mg were homogenized in 300–400 µL cold lysis buffer (methanol:water:chloroform, 9:1:1, −20°C) with a Precellys 24 Homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France), according to standard protocols. Tissue extracts were centrifuged for 10 min (15,000 g, 4°C) and supernatants (150 µL) were collected in microcentrifuge tubes. Serum aliquots of 100 µL were mixed with 1 mL cold solvent (acetonitrile:2-propanol:water, 3:2:2, −20°C) in microcentrifuge tubes, vortexed and centrifuged for 10 min (15,000 g, 4°C). Both tissue and serum sample were then dried at 40°C in Techne DB3 Dri-Block® heater (Bibby Scientific Ltd., Stone, UK). On the day of analysis, dried extracts were resuspended in 300 µL of methanol and split in equal parts for liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS) analyses. GC-MS samples were transferred into glass tubes, and 10 µL aliquots were destined to quality control (QC) before evaporation and chemical derivatization. LC-MS samples were dried again, resuspended in 300 µL water and split as follows: 2 × 50 µL aliquots were transferred to HPLC vials for targeted (LC-QQQ) and profiling (LC-QTOF) analysis, 1 × 10 µL aliquot was destined to QC, and the remaining material was kept as back-up. For all analyses, acquisition was performed randomly alongside 5 QC samples injected at regular intervals. The instrument was calibrated daily with pre-validated standard solutions and the auto-tuning function.
Targeted analysis by GC coupled to triple quadrupole (QQQ) mass spectrometry
Dried extracts were dissolved in 50 µL of pyridine (Sigma-Aldrich, Saint Louis, MO, US) supplemented with 20 mg/L methoxyamine hydrochloride (Sigma-Aldrich) and left at room temperature in the dark. Sixteen h later, 50 µL N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA, Sigma-Aldrich) were added to the samples and the latter were incubated for 30 min at 40°C. GC studies were conducted as previously described.Citation37 GC-MS/MS acquisitions were performed on a 7890A gas chromatograph coupled to a triple quadrupole 7000A detector (both from Agilent Technologies, Santa Clara, CA, US), equipped with an electronic impact source (EIS) operating in positive mode and a 30 m × 0.25 mm I.D. × 0.25 μm film thickness HP-5MS capillary column (Agilent Technologies). Sample aliquots of 1 µL were injected into an inlet operating in splitless mode and set at 250°C. Helium gas flow rate was set at 1 mL/min and the septum purge flow at 3 mL/min. The temperature was programmed as follows: 60°C for 1 min, +10°C/min up to 210°C, hold for 3 min, +5°C/min up to 230°C, +15°C/min up to 325°C and hold for 5 min. The transfer line and ion-source temperature was 250°C and 230°C, respectively. The duty cycle was 39 min. In all, 267 MRM transitions corresponding to 118 analytes were quantified with the MassHunter Quantitative Analysis software (Agilent Technologies, B.05.00), and results were exported to the R statistical environment for data reduction and statistical analyses.
Untargeted analysis by UHPLC coupled to quadrupole-time of flight (QTOF) mass spectrometry
Profiling of tissue/serum metabolites was performed on a RRLC 1260 system (Agilent Technologies) coupled to a QTOF 6520 detector (Agilent Technologies), equipped with an electrospray source operating in full scan mode, from 50 to 1,000 Da for both positive and negative ionization modes. The gas temperature was set at 350°C, gas flow of 12 L/min, capillary voltage at 3.5 kV and fragmentor voltage at 120 V. Two reference masses were used to maintain the mass accuracy during analysis: m/z 121.050873 and m/z 922.009798 in positive mode, and m/z 112.985587 and m/z 980.016375 in negative mode. Sample aliquots of 10 μL were injected on a Sb-Aq column (100 mm × 2.1 mm, particle size 1.8 μm, Agilent Technologies), protected by a XDB-C18 guard column (5 mm × 2.1 mm, particle size 1.8 μm, Agilent Technologies) and heated at 40°C. The gradient mobile phase consisted of 0.2% acetic acid (v:v in water) (A) and acetonitrile (B). The flow rate was set at 0.3 mL/min. Initial condition was 98% phase A and 2% phase B and the gradient changes as follows: from 2% to 95% phase B in 7 min, 95% phase B for 3 min, and equilibration with 2% phase B for 3 min. The autosampler was kept at 4°C. Profiling data were treated as described below.
Targeted analysis by UHPLC coupled to triple quadrupole (QQQ) mass spectrometry
Targeted analysis was performed on a RRLC 1260 system coupled to a Triple Quadrupole 6410 detector (Agilent Technologies), equipped with an electrospray source operating in positive mode. Gas temperature was set at 350°C, gas flow at 12 L/min, and capillary voltage at 3.5 kV. Sample aliquots of 10 μL were injected on a Zorbax Eclipse XDB-C18 column (100 mm × 2.1 mm, particle size 1.8 μm, Agilent Technologies), protected by a XDB-C18 guard column (5 mm × 2.1 mm, particle size 1.8 μm) and heated at 40°C. The gradient mobile phase consisted of 2 mM of dibutyl ammonium acetate (DBAA) in water (A) and acetonitrile (B). The flow rate was set at 0.2 mL/min, and the gradient changed as follows: initial condition (90% phase A and 10% phase B) was maintained for 4 min, from 10% to 95% phase B over 3 min. The column was washed using 95% mobile phase B for 3 min and equilibrated using 10% phase B for 3 min. The autosampler was kept at 4°C. Target and qualified MRM transitions corresponding to 13 metabolites (adenosine, AMP, ADP, ATP, NAD, NADP, NADH, NADPH, FAD, acetyl-CoA, malonyl-CoA, succinyl-CoA and coenzyme A) were quantified with the MassHunter Quantitative Analysis software (B.04.00) and the results were exported to the R statistical environment for data reduction and statistical analyses.
Signal processing for LC-QTOF profiles
Profiles generated by LC-QTOF were processed using an in-house set of tools that convert raw MS data into a matrix compatible with statistical analysis. Raw data files were treated with the Molecular Feature Extraction (MFE) algorithm of the MassHunter Quantitative Analysis software, in order to identity predominant ions in form of triplets [mass to charge ratio (m/z); retention time (RT); intensity]. Ions (1) that were flagged as isotopes by the MFE algorithm, (2) that had a mass defect between 0.75 and 0.95, (3) with a signal intensity below 3,000, and (4) outside the 0.8–8 min RT range were discarded from downstream processing. To circumvent the elevated false negative rate achieved by the vendor MFE algorithm, in-house scripts were used to extract, filter, align and integrate the ion chromatograms from the MFE feature lists. In short: (1) feature lists were roughly grouped across all the samples; (2) for each cluster, extracted ion chromatograms (EICs) were generated with a tolerance of 16 ppm and 0.5 min; (3) EICs of poor quality (spikes and/or high frequency noise) or detected in less than 2 samples were excluded; (4) high-quality EICs found in the QC samples were employed to perform time domain alignment; and (5) peaks were identified and integrated across all samples. In the datasets recorded in the positive and negative modes, 6,998 unique features were detected and sent to annotation and post-processing.
Peak annotation for LC-QTOF profiles
Peaks detected by LC-QTOF were classified in 3 annotation categories after interrogating our in-house annotation database MetIGR with each peak feature m/z and RT. MetIGR aggregates chemical entities taken from KEGG, HMDB, LipidMaps, ChEBI and MetaCyc and implemented as previously described by Draper et al.,Citation38 and contains information related to biochemical pathways, drug modes of action, several ontologies as well as the analytical characteristics of >200 standards of pure compounds measured in the exact conditions as the experimental samples were. So-called “annotated” peaks have their identity provisionally confirmed by matching both accurate mass and RT (within 17 ppm and 10 sec windows) to a chemical standard. Please note that the RT for most acyl-carnitines and fatty acids were inferred from their respective number of carbons and double bonds. The accurate mass of so-called “putative” features matches (±17 ppm) either the deprotonated ([M-H]−) or the protonated ([M+H]+) ionization product of a MetIGR entry that is recorded as endogenous in HMDB, with a biological role in KEGG/BRITE or employed in RECON. Finally, “profiling” features were the remaining signals found by the deconvolution algorithm. The origin of these features is largely unknown. Based on accurate mass, some of the profiling features might (1) have matched an entry in our library of standards but failed the RT criterion, (2) have matched an entry in MetIGR that is not classified as a molecule of potential interest, or (3) be a less frequent ionization product of a MetIGR entry. LC-QTOF profile data are presented as follows: annotated compounds are denoted with their common name, putative features with the empirical formula of the metabolite in its neutral form, and profiling features with their accurate mass. For the 3 categories, names are followed by the retention time and measurement conditions (i.e., positive, p or negative, n).
Data collation and normalization
Peaks extracted from LC-QTOF profiling or targeted studies were gathered and further reduced before data analysis and interpretation. Feature selection criteria were computed for each tissue separately and included: missing value rate below 25%, 95% signal to blank ratio (SBR)>5, coefficient of variation from pooled-QC samples lower than 20%. SBR was defined as the ratio between the height of a peak in a sample and the height of the same peak in blank sample. Nominal intensity values were applied when peaks were missing or too weak in blank samples (LC-QTOF, 500; LC-QQQ, 10; GC-QQQ, 150). Priority was given to the targeted method over profiling to discard redundant metabolites, and to the ionization mode producing the most abundant ions to select an annotated or putative feature that was detected in both profiling modes. The final metabolomics matrix comprised 2,598 features of which 304 were non-redundant, putatively assigned metabolites, and 211 were unique, precisely identified metabolites. The distribution of these features in samples and annotation are given in Table S1. All statistical analyses and data representation were performed on pre-processed, log2-transformed and imputed data,Citation39 and reported as such without back-transformation. Supervised LDA as implemented in FIEmsproCitation40 was used to generate discriminant functions that best describe separation between samples. Moderated statistics were used for differential analysis.Citation41 Fold changes and associated p values are reported in Table S1 alongside metabolite name, HMDB and KEGG IDs for facilitating data exchange. Estimated parameters are accompanied by their 95% confidence intervals. p values are 2-tailed and considered significant when ≤ 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1064206_Supplements.zip
Download Zip (1.1 MB)Funding
GK is supported by the Ligue contre le Cancer (équipe labelisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Fondation Bettencourt-Schueller; Fondation de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). F.M. is grateful to the FWF for grants LIPOTOX, I1000, P23490-B12, and P24381-B20 and the BMWFW for grant “Unconventional research.”
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Berraaouan A, Abid S, Bnouham M. Antidiabetic oils. Curr Diabetes Rev 2013; 9:499-505; PMID:24111621; http://dx.doi.org/10.2174/15733998113096660081
- Fattore E, Fanelli R. Palm oil and palmitic acid: a review on cardiovascular effects and carcinogenicity. Int J Food Sci Nutr 2013; 64:648-59; PMID:23406428; http://dx.doi.org/10.3109/09637486.2013.768213
- Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med 2015; 128:229-38; PMID:25447615; http://dx.doi.org/10.1016/j.amjmed.2014.10.014
- Sales-Campos H, Souza PR, Peghini BC, da Silva JS, Cardoso CR. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev Med Chem 2013; 13:201-10; PMID:23278117
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008; 132:27-42; PMID:18191218; http://dx.doi.org/10.1016/j.cell.2007.12.018
- Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell 2010; 40:280-93; PMID:20965422; http://dx.doi.org/10.1016/j.molcel.2010.09.023
- Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 2012; 11:709-30; PMID:22935804; http://dx.doi.org/10.1038/nrd3802
- Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V, et al. Autophagy in malignant transformation and cancer progression. EMBO J 2015; 34:856-80; PMID:25712477; http://dx.doi.org/10.15252/embj.201490784
- Niso-Santano M, Malik SA, Pietrocola F, Bravo-San Pedro JM, Marino G, Cianfanelli V, Ben-Younès A, Troncoso R, Markaki M, Sica V, et al. Unsaturated fatty acids induce non-canonical autophagy. EMBO J 2015; 34:1025-41; PMID:25586377; http://dx.doi.org/10.15252/embj.201489363
- Niso-Santano M, Bravo-San Pedro JM, Maiuri MC, Tavernarakis N, Cecconi F, Madeo F, Codogno P, Galluzzi L, Kroemer G. Novel inducers of BECN1-independent autophagy: cis-unsaturated fatty acids. Autophagy 2015; 11:575-7; PMID:25714112; http://dx.doi.org/10.1080/15548627.2015.1017222
- Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex–at the crossroads of autophagy and beyond. Trends Cell Biol 2010; 20:355-62; PMID:20356743; http://dx.doi.org/10.1016/j.tcb.2010.03.002
- Djavaheri-Mergny M, Maiuri MC, Kroemer G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene 2010; 29:1717-9; PMID:20101204; http://dx.doi.org/10.1038/onc.2009.519
- Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol 2012; 13:7-12.
- Griffiths WJ, Koal T, Wang Y, Kohl M, Enot DP, Deigner HP. Targeted metabolomics for biomarker discovery. Angew Chem Int Ed Engl 2010; 49:5426-45; PMID:20629054; http://dx.doi.org/10.1002/anie.200905579
- Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature 2015; 517:302-10; PMID:25592535; http://dx.doi.org/10.1038/nature14190
- Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell 2014; 159:1263-76; PMID:25480292; http://dx.doi.org/10.1016/j.cell.2014.11.006
- Russell RC, Yuan HX, Guan KL. Autophagy regulation by nutrient signaling. Cell Res 2014; 24:42-57; PMID:24343578; http://dx.doi.org/10.1038/cr.2013.166
- Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell 2011; 146:682-95; PMID:21884931; http://dx.doi.org/10.1016/j.cell.2011.07.030
- Morselli E, Marino G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Bénit P, Rustin P, Criollo A, et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol 2011; 192:615-29; PMID:21339330; http://dx.doi.org/10.1083/jcb.201008167
- Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 2009; 11:1305-14; PMID:19801973; http://dx.doi.org/10.1038/ncb1975
- Morgan DM. Polyamines. An introduction. Methods Mol Biol 1998; 79:3-30; PMID:9463813
- Marino G, Pietrocola F, Eisenberg T, Kong Y, Malik SA, Andryushkova A, Schroeder S, Pendl T, Harger A, Niso-Santano M, et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell 2014; 53:710-25; PMID:24560926; http://dx.doi.org/10.1016/j.molcel.2014.01.016
- Eisenberg T, Schroeder S, Andryushkova A, Pendl T, Kuttner V, Bhukel A, Mariño G, Pietrocola F, Harger A, Zimmermann A, et al. Nucleocytosolic depletion of the energy metabolite acetyl-coenzyme a stimulates autophagy and prolongs lifespan. Cell Metab 2014; 19:431-44; PMID:24606900; http://dx.doi.org/10.1016/j.cmet.2014.02.010
- Marino G, Pietrocola F, Kong Y, Eisenberg T, Hill JA, Madeo F, Kroemer G. Dimethyl alpha-ketoglutarate inhibits maladaptive autophagy in pressure overload-induced cardiomyopathy. Autophagy 2014; 10:930-2; PMID:24675140; http://dx.doi.org/10.4161/auto.28235
- Schroeder S, Pendl T, Zimmermann A, Eisenberg T, Carmona-Gutierrez D, Ruckenstuhl C, Mariño G, Pietrocola F, Harger A, Magnes C, et al. Acetyl-coenzyme A: a metabolic master regulator of autophagy and longevity. Autophagy 2014; 10:1335-7; PMID:24904996; http://dx.doi.org/10.4161/auto.28919
- Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab 2014; 25:42-52; PMID:24140022; http://dx.doi.org/10.1016/j.tem.2013.09.002
- Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY) 2011; 3:716-32; PMID:21869457
- Morselli E, Galluzzi L, Kepp O, Criollo A, Maiuri MC, Tavernarakis N, Madeo F, Kroemer G. Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol. Aging (Albany NY) 2009; 1:961-70; PMID:20157579
- Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol 2010; 12:842-6; PMID:20811357; http://dx.doi.org/10.1038/ncb0910-842
- Minois N, Carmona-Gutierrez D, Bauer MA, Rockenfeller P, Eisenberg T, Brandhorst S, Sigrist SJ, Kroemer G, Madeo F. Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell Death Dis 2012; 3:e401; PMID:23059820; http://dx.doi.org/10.1038/cddis.2012.139
- Buttner S, Broeskamp F, Sommer C, Markaki M, Habernig L, Alavian-Ghavanini A, Carmona-Gutierrez D, Eisenberg T, Michael E, Kroemer G, et al. Spermidine protects against alpha-synuclein neurotoxicity. Cell Cycle 2014; 13:3903-8; PMID:25483063; http://dx.doi.org/10.4161/15384101.2014.973309
- Pietrocola F, Lachkar S, Enot DP, Niso-Santano M, Bravo-San Pedro JM, Sica V, Izzo V, Maiuri MC, Madeo F, Mariño G, et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ 2015; 22:509-16; PMID:25526088; http://dx.doi.org/10.1038/cdd.2014.215
- Harrison DH, Newton J. Two flaps to resurface the basal flexion-crease of the finger area. J Hand Surg Br 1991; 16:78-83; PMID:2007822; http://dx.doi.org/10.1016/0266-7681(91)90135-B
- Sinclair DA, Guarente L. Small-molecule allosteric activators of sirtuins. Annu Rev Pharmacol Toxicol 2014; 54:363-80; PMID:24160699; http://dx.doi.org/10.1146/annurev-pharmtox-010611-134657
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153:1194-217; PMID:23746838; http://dx.doi.org/10.1016/j.cell.2013.05.039
- Riera CE, Dillin A. Tipping the metabolic scales towards increased longevity in mammals. Nat Cell Biol 2015; 17:196-203; PMID:25720959; http://dx.doi.org/10.1038/ncb3107
- Tsugawa H, Tsujimoto Y, Sugitate K, Sakui N, Nishiumi S, Bamba T, Fukusaki E. Highly sensitive and selective analysis of widely targeted metabolomics using gas chromatography/triple-quadrupole mass spectrometry. J Biosci Bioeng 2014; 117:122-8; PMID:23867096; http://dx.doi.org/10.1016/j.jbiosc.2013.06.009
- Draper J, Enot DP, Parker D, Beckmann M, Snowdon S, Lin W, Zubair H. Metabolite signal identification in accurate mass metabolomics data with MZedDB, an interactive m/z annotation tool utilising predicted ionisation behaviour 'rules'. BMC Bioinformatics 2009; 10:227; PMID:19622150; http://dx.doi.org/10.1186/1471-2105-10-227
- Kim H, Golub GH, Park H. Missing value estimation for DNA microarray gene expression data: local least squares imputation. Bioinformatics 2005; 21:187-98; PMID:15333461; http://dx.doi.org/10.1093/bioinformatics/bth499
- Enot DP, Lin W, Beckmann M, Parker D, Overy DP, Draper J. Preprocessing, classification modeling and feature selection using flow injection electrospray mass spectrometry metabolite fingerprint data. Nat Protoc 2008; 3:446-70; PMID:18323816; http://dx.doi.org/10.1038/nprot.2007.511
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43:e47; PMID:25605792; http://dx.doi.org/10.1093/nar/gkv007
