Abstract
Ectopic mineralization disorders inflicting the connective tissues display a spectrum of severity, some developing in utero and being diagnosed by prenatal ultrasound. This study was designed to test the hypothesis that the mineral content of maternal diet can influence the mineralization in the offspring. Pregnant Abcc6−/− mice, on 2 different strain backgrounds, were maintained either on normal rodent diet or on “acceleration diet,” rich in phosphate and low in magnesium, which has been previously shown to enhance the mineralization processes. The offspring were examined for mineralization by histopathology of various tissues and quantitated by chemical assay of calcium. The ectopic mineralization in the dermal sheath of vibrissae, a progressive biomarker of the overall mineralization, was readily detectable at the age of 4 weeks in the pups whose mothers were on the acceleration diet, while no evidence of mineralization was noted in those on normal diet. The mineralization of the vibrissae progressively increased when examined at 12 weeks of age. There was a significant reduction in urinary calcium and significant increase in urinary phosphorus concentrations both at 4 and 12 weeks of age in mice on the acceleration diet as compared to those on control diet. The results demonstrate that the mineral content of the maternal diet can influence ectopic mineralization in the offspring of mice genetically predisposed to ectopic mineralization (Abcc6−/−). These observations have implications for dietary management of pregnancies in which the fetus is diagnosed by prenatal ultrasound to have an ectopic mineralization disorder.
Abbreviations
| ACDC | = | arterial calcification due to CD73 deficiency |
| GACI | = | generalized arterial calcification of infancy |
| PXE | = | pseudoxanthoma elasticum. |
Introduction
Ectopic soft tissue mineralization, particularly when inflicting the cardiovascular system, is a major cause of morbidity and mortality.Citation1,2 The processes culminating in calcium phosphate complex deposition in extracellular matrices of connective tissue are complex and influenced by the individuals' genetic background, nutritional status, and lifestyle variables, including diet. Critical insight into the ectopic mineralization processes has been achieved through observations on a group of heritable disorders demonstrating premature vascular mineralization.Citation2,3 The prototype of such conditions is pseudoxanthoma elasticum (PXE), a multisystem disorder characterized by mineralization of arterial blood vessels resulting in development of cardiovascular complications, including nephrogenic hypertension, intermittent claudication, bleeding from the intestinal blood vessels, as well as early myocardial infarcts and strokes.Citation4,5 Calcium hydroxyapatite deposition also occurs in the skin and retina, causing premature aging appearance as well as progressive loss of visual acuity and blindness, respectively.
PXE is caused by mutations in the ABCC6 gene, which encodes a putative efflux transporter, ABCC6, primarily expressed in the liver and the kidneys.Citation4 While the precise physiologic role of ABCC6 is currently unclear, it has been suggested to participate in release of intracellular ATP to the extracellular milieu.Citation6,7 Clinically, PXE is characterized by late onset, the diagnosis frequently being made in mid-teens, but PXE has also been encountered in early infancy, diagnosed as pediatric PXE. Another condition, generalized arterial calcification of infancy (GACI), is a severe disease manifesting with extensive, early-onset vascular calcification, frequently diagnosed by prenatal ultrasound.Citation3,8 The cardiovascular involvement of mineralization results in complications usually leading to early demise of the affected individuals during the first year of life. In most cases, GACI is caused by mutations in the ENPP1 gene encoding an enzyme, ectonucleotide pyrophosphatase/phosphodiesterase1 (ENPP1), which catalyzes conversion of ATP to AMP and inorganic pyrophosphate (PPi), the latter being a powerful physiologic inhibitor of mineralization.Citation9 In fact, proper PPi/Pi ratio is required for prevention of spontaneous calcium phosphate crystal deposition under normal homeostatic conditions. In addition to mutations in the ENPP1 gene, recent studies have demonstrated that a subgroup of patients with GACI harbor mutations in the ABCC6 gene, many of them being the same as those found in patients with classic PXE.Citation10,11 Finally, another ectopic mineralization disorder, arterial calcification due to CD73 deficiency (ACDC), manifests with mineralization of lower-extremity arteries as well as hand and foot joint-capsules.Citation12-14 This disease is caused by mutations in the NT5E gene which encodes an enzyme, CD73, converting 5′-AMP to adenosine and Pi. Thus, there is a spectrum of ectopic mineralization disorders with overlapping phenotypic features, which involve mutations in the genes responsible for the maintenance of PPi/Pi physiologic ratios.
There are several lines of evidence suggesting that diet, particularly with respect to its mineral content, may modify the severity of ectopic mineralization. Early on, retrospective observations suggested that excessive ingestion of dairy products, rich in calcium and phosphate, during childhood and adolescence, results in more severe phenotypic presentation of PXE later in life.Citation15,16 More recently, several studies using transgenic mice with targeted ablation of the Abcc6 or Enpp1 gene as a model for PXE and GACI, respectively, have demonstrated that the mineral content of diet of these mice can alter the extent of ectopic mineralization. Specifically, increased levels of magnesium in the diet, 5 times over the standard rodent diet, completely abolished the ectopic mineralization in these mice.Citation17,18 At the same time, addition of phosphate, 2 times over the standard diet, when combined with reduced (20%) magnesium content, significantly accelerated the mineralization; this diet has been designated as the “acceleration diet.”Citation19-21
As indicated above, the diagnosis of GACI is frequently made during pregnancy through prenatal ultrasound. Thus, we have now tested the hypothesis that the mineral content of the maternal diet may influence the degree of mineralization in the offspring in Abcc6 knockout mice. Since the degree of mineralization has been previously shown to be dependent on the mouse strain background,Citation22 in this study we fed Abcc6 knockout mice on 2 different genetic backgrounds (C57BL/6J and 129S1/SvImJ) with the acceleration diet during the entire pregnancy. The extent of mineralization was then determined in the offspring at 4 and 12 weeks of age by histopathological analysis and by direct quantitative chemical assay of calcium.
Results
The mouse model for ectopic mineralization
We have previously demonstrated that Abcc6−/− mice develop ectopic mineralization, the first site of mineralization being the dermal sheath of vibrissae, a connective tissue capsule that surrounds the hair bulb of vibrissae in the muzzle skin.Citation23,24 Mineralization of vibrissae is first documented around 5–6 weeks of age when the mice are kept on standard rodent diet. The mineralization then progressively increases, reflecting overall mineralization of the internal organs in these mice, and consequently, the vibrissae mineralization serves as a quantifiable biomarker of the mineralization process. We have also demonstrated that feeding these mice with “acceleration diet,” enriched in phosphate and low in magnesium, increases the degree of mineralization.Citation19 In this study, pregnant Abcc6−/− mice on 2 different strain backgrounds, C57BL/6J and 129S1/SvImJ, were kept either on the acceleration diet or normal rodent diet during the entire pregnancy and the subsequent postpartum period, and the degree of mineralization in the offspring was determined first by muzzle skin biopsy at 4 weeks of age. After weaning, some pups were continued either on acceleration diet or on control diet up to 12 weeks of age ()
Table 1. Experimental groups of Abcc6−/− mice by maternal diet and strain background*
Maternal diet influences ectopic mineralization in the offspring
The mineralization was determined in biopsies of the muzzle skin of the offspring by Alizarin red stain (). Evidence of mineralization was clearly noted in offspring at 4 weeks of age on both mouse strains when the mother was kept on acceleration diet (), while no mineralization was noted in the corresponding mice kept on normal diet (). At the time of weaning the pups were placed on acceleration diet, and progressively increased mineralization was noted when examined at 12 weeks of age ().
Figure 1. Histopathologic demonstration of ectopic mineralization in the dermal sheath of vibrissae, a connective tissue capsule surrounding the hair bulb in the muzzle skin (a), or in various internal organs and in the eyes of Abcc6−/− mice (b). (a) Pregnant Abcc6−/− mice were kept on acceleration diet rich in phosphate and low in magnesium (Groups A, B, E, and F) or on normal standard rodent diet (Groups C, D, G, and H). Muzzle skin biopsies were obtained in the newborn mice at 4 weeks (Groups A, C, E, and G) or at 12 weeks of age (Groups B, D, F, and H). The mice were congenic either on C57BL/6J (Groups A-D) or 129S1/SvImJ (Groups E-H) strain background. (b) Extensive mineralization of the dermal sheath of vibrissae of mice on acceleration diet, as shown in Group B, was accompanied by mineralization in the aorta, arterial blood vessels in the liver, kidney and heart, as well as in the retina of the eye. For designation of the treatment groups, see . (Alizarin red stain; representative panels of 7–9 mice in each group).
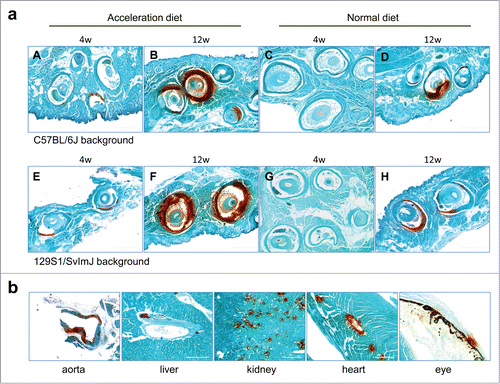
The extent of mineralization was quantitated by chemical assay of calcium which revealed that at each time point, the mice on 129S1/SvImJ background had higher levels of mineralization (). The high level of mineralization of vibrissae in these mice was accompanied by extensive mineralization in the aorta, arterial blood vessels in the liver, kidney and heart, as well as in the retina at 12 weeks of age (). The number of mice in each group with readily detectable mineralization, as determined by histopathology with H&E staining, is shown in . The number of mice with mineralization in different organs increased from 4 weeks to 12 weeks, and there was a higher degree of mineralization when the mice were placed on acceleration diet. At 4 week time point, the pups with mothers on acceleration diet had a higher number of mineralization in the vibrissae, kidney and heart than those on control diet (). Mineralization of aorta was noted only at 12 weeks of age of pups with mothers on acceleration diet. Thus, the results clearly indicate that changes in the mineral content of the maternal diet influence the degree of mineralization in newborn pups with targeted ablation of the Abcc6 gene. In addition, the genetic background of mice influences such mineralization.
Figure 2. Quantitation of the ectopic mineralization in the muzzle skin containing the dermal sheath of vibrissae by direct chemical assay of calcium. The pregnant mothers were kept either on acceleration diet or on normal rodent diet, and the pups representing 2 different strain backgrounds as indicated, were examined at 4 and 12 weeks of age. For designation of the treatment groups, see . (The values are mean + SE, n = 7–9 mice per group; statistical analysis was performed with Student's 2-tailed t-test).
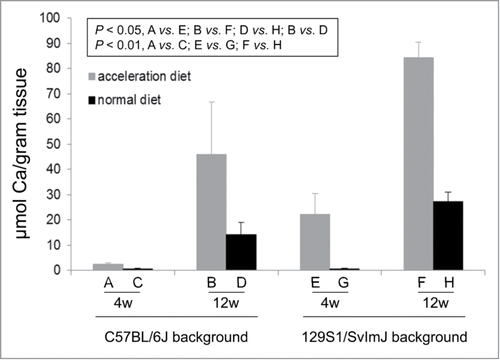
Table 2. Aberrant tissue mineralization in Abcc6−/− mice in different groups*
Assay of serum and urinary calcium and phosphate
To gain insight into the potential mechanisms how the acceleration diet might enhance the ectopic mineralization, calcium and phosphorus concentrations in the serum and urine of mice was determined. No significant changes in the calcium or phosphorus levels in the serum were noted, and the Ca/P ratio remained in the same range in all groups (). In contrast, the concentration of calcium in the urine was significantly reduced and the concentration of phosphorus was markedly increased both at 4 week and 12 week time points in mice kept on acceleration diet. These observations suggest that the Abcc6−/− mice retain a net balance of calcium which then precipitates in the form of calcium hydroxyapatite on the connective tissue matrices in different tissues.
Table 3. Calcium and phosphorus concentrations in the serum and urine of mice*
Discussion
GACI and PXE, in their classic forms, are 2 clinically distinct autosomal recessive disorders. GACI manifests with extensive arterial calcification at birth leading to early demise of the affected individuals, while the clinical presentations of PXE are of late-onset and slowly progressive.Citation8 PXE is caused by mutations in the ABCC6 gene, while most patients with GACI have biallelic mutations in the ENPP1 gene. However, several families with characteristic features of GACI have been recently shown to harbor mutations in the ABCC6 gene, and surprisingly, many of these mutations have been previously shown to cause the classic form of PXE in unrelated families.Citation10,11 ABCC6 encodes a putative transmembrane transporter protein, ABCC6, expressed primarily in the baso-lateral surface of hepatocytes, at low levels in the kidneys and the intestine, but is essentially undetectable in tissues directly affected by mineralization. The precise mechanisms of peripheral vascular and soft tissue calcification in patients with GACI and PXE due to mutations in the ABCC6 are currently unknown.
Recent studies have focused on the role of the mineral content of diet in modifying the severity of PXE and GACI. Original retrospective surveys suggested that those patients with PXE who had a history of high intake of dairy products, rich in calcium and phosphate, during childhood and adolescence developed more severe disease later in life.Citation15,16 More recently, genetically controlled studies utilizing the Abcc6−/− mouse as a preclinical platform have specifically shown that magnesium content of the diet can influence the extent of ectopic mineralization in peripheral tissues. Specifically, supplementation of the mouse diet with magnesium in amounts that increase the content by 5-fold over the standard diet completely abolished the mineralization noted in the Abcc6−/− mice.Citation17,18 Conversely, the experimental diet with reduced magnesium and elevated phosphorus content was shown to accelerate the mineralization process, accompanied by marked decrease in the urinary output of calcium and concomitant increase in phosphate in the treated mice.Citation19 These findings support the notion that changes in the diet, specifically increase in dietary magnesium, might be helpful for patients with PXE. In this context, it should be noted that various vegetables (spinach, tomato and pumpkin seeds) and grains (buckwheat flour, oat bran, barley and wheat flour) are particularly rich in magnesium.Citation25 Based on these preclinical mouse studies, a double-blinded clinical trial of PXE patients with a magnesium-enriched experimental diet is underway (http://clinicaltrials.gov/show/NCT01525875, last verified January 2015). Finally, preliminary studies on oral phosphate binders have suggested an improvement in the degree of skin manifestations of patients with PXE, and these studies are being extended to larger cohorts of patients.Citation26,27
In this study, we characterized the effect of the mineral content of the maternal diet on the onset and severity of ectopic mineralization in the offspring, utilizing the ectopic mineralization in the dermal sheath of vibrissae as a progressive biomarker of the overall mineralization process in the Abcc6−/− mice. The results indicated that when pregnant mothers were placed on standard rodent diet, vibrissae mineralization was not noted until 5–6 weeks of age in the offspring. However, evidence of mineralization was clearly noted in offspring as early as 4 weeks of age when the mother was placed on “acceleration diet,” an experimental diet with increased phosphorus and reduced magnesium content that has been previously shown to accelerate the mineralization process. Mineralization progressively increased in the offspring when examined at 12 weeks of age. In addition, the Abcc6−/− mice on 129S1/SvImJ background showed significantly higher levels of mineralization as compared to mice on C57BL/6J strain background, suggesting a role for modifier genes in influencing the extent of mineralization.
The diagnosis of GACI is often made by prenatal ultrasound during pregnancy. Identification of mutations in the ABCC6 gene can be used for confirmation of the clinical diagnosis, carrier detection and presymptomatic identification of affected individuals with family history of PXE/GACI. Early diagnosis by mutation analysis from chorionic villus sampling is also possible for families with affected individuals. Our observations have implications for the clinical management of patients with PXE and GACI caused by ABCC6 mutations. Specifically, for patients with family history, as soon as the clinical diagnosis and mutation analysis have been made during pregnancy, a combination of therapies, including appropriate maternal diet with modified mineral content and possibly supplementation with bisphosphonates,Citation28 might slow down the progression of PXE/GACI in the fetus and improve the quality of postnatal life of patients with these, currently intractable, diseases. These approaches may also be applicable to other ectopic mineralization disorders affecting the skin and the cardiovascular system, such as ACDC and familial tumoral calcinosis.
Materials and Methods
Mice
Abcc6tm1JfK mice (referred to in this study as Abcc6−/− mice) were developed by targeted ablation of the mouse Abcc6 gene.Citation23 These mice were made congenic on C57BL/6J or 129S1/SvImJ background (10 generations each). Mice were placed on either normal rodent diet (Diet 5010; PMI Nutritional International, Brentwood, MO) or “acceleration diet” (Diet TD.00442; Harlan Teklad, Madison, WI) during pregnancy and postnatally. For different groups, see . The acceleration diet is enriched in phosphorus (2X) and has reduced magnesium content (20%) in comparison to the normal rodent diet. For the specific contents of the 2 diets see, normal diet: http://www.labdiet.com/cs/groups/lolweb/@labdiet/documents/web_content/mdrf/mdi4/∼edisp/ducm04_028443.pdf; and acceleration diet: http://www.harlan.com/products_and_services/research_models_and_services/tekladdiets. This study was approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University. The offspring were euthanized at either 4 or 12 weeks of age for tissue analysis.
Histopathology
Biopsies from muzzle skin containing vibrissae as well as the internal organs were fixed in 10% phosphate-buffered formalin and embedded in paraffin. Paraffin sections (6 µm) were stained with hematoxylin-eosin (H&E) and Alizarin red stains using standard methods.
Chemical analyses
To quantify the calcium deposition, muzzle skin biopsies containing vibrissae were obtained and decalcified with 0.15 N HCl for 48 h at room temperature. The calcium content was determined colorimetrically by the σ-cresolphthalein complexone method (Calcium (CPC) Liquicolor; Stanbio Laboratory, Boerne, TX). The values for calcium were normalized to tissue weight. Calcium in the serum and urine samples was quantitatively assayed as above. The serum and urinary phosphate content was determined with a Malachite Green Phosphate Assay kit (BioAssay Systems, Hayward, CA).
Statistical analysis
The results in different groups of mice were evaluated by Student's 2-tailed t-test. Fisher's Exact test with exact logistic regression method was used to determine the significance between the proportional differences in mineralization in different groups. Statistical significance was reached with P < 0.05. Analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dian Wang and Jieyu Zhang for technical help.
Funding
Dr. Tingting Zhan provided assistance in statistical analyses (supported by the Sidney Kimmel Cancer Center grant P30CA056036 from the NIH/NCI). Carol Kelly assisted in manuscript preparation. Supported by NIH/NIAMS grants K01AR064766 (QL) and R01AR055225 (JU).
References
- Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007; 49:1860-70; PMID:17481445; http://dx.doi.org/10.1016/j.jacc.2006.10.079
- Li Q, Uitto J. Mineralization/anti-mineralization networks in the skin and vascular connective tissues. Am J Pathol 2013; 183:10-8; PMID:23665350; http://dx.doi.org/10.1016/j.ajpath.2013.03.002
- Nitschke Y, Rutsch F. Genetics in arterial calcification: lessons learned from rare diseases. Trends Cardiovasc Med 2012; 22:145-9; PMID:23122642; http://dx.doi.org/10.1016/j.tcm.2012.07.011
- Uitto J, Li Q, Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. J Invest Dermatol 2010; 130:661-70; PMID:20032990; http://dx.doi.org/10.1038/jid.2009.411
- Uitto J, Jiang Q, Varadi A, Bercovitch LG, Terry SF. Pseudoxanthoma elasticum: Diagnostic features, classification and treatment options. Expert Opin Orphan Drugs 2014; 2:567-77; PMID:25383264; http://dx.doi.org/10.1517/21678707.2014.908702
- Jansen RS, Kucukosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IE, Bergen AA, Gorgels TG, Borst P, van de Wetering K. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Nat Acad Sci USA 2013; 110:20206-11; PMID:24277820; http://dx.doi.org/10.1073/pnas.1319582110
- Jansen RS, Duijst S, Mahakena S, Sommer D, Szeri F, Varadi A, Plomp A, Bergen AA, Oude Elferink RP, Borst P, et al: ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol 2014; 34:1985-9; PMID:24969777; http://dx.doi.org/10.1161/ATVBAHA.114.304017
- Nitschke Y and Rutsch F. Generalized arterial calcification of infancy and pseudoxanthoma elasticum: two sides of the same coin. Front Genet 2012; 3:302; PMID:23269929; http://dx.doi.org/10.3389/fgene.2012.00302
- Ruf N, Uhlenberg B, Terkeltaub R, Nurnberg P and Rutsch F. The mutational spectrum of ENPP1 as arising after the analysis of 23 unrelated patients with generalized arterial calcification of infancy (GACI). Hum Mutat 2005; 25:98; PMID:15605415; http://dx.doi.org/10.1002/humu.9297
- Nitschke Y, Baujat G, Botschen U, Wittkampf T, du Moulin M, Stella J, Le Merrer M, Guest G, Lambot K, Tazarourte-Pinturier MF, et al: Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet 2012; 90:25-39; PMID:22209248; http://dx.doi.org/10.1016/j.ajhg.2011.11.020
- Li Q, Brodsky JL, Conlin L, Pawel B, Glatz A, Gafni RI, Schurgers LJ, Uitto J, Hakonarson H, Deardoff MA, et al: Mutations in the ABCC6 gene as a cause of generalized arterial calcification of infancy: Genotypic overlap with pseudoxanthoma elasticum. J Invest Dermatol 2014; 134:658-65; PMID:24008425; http://dx.doi.org/10.1038/jid.2013.370
- Li Q, Price TP, Sundberg JP and Uitto J. Juxta-articular joint-capsule mineralization in CD73 deficient mice: Similarities to patients with NT5E mutations. Cell Cycle 2014; 13:2609-15; PMID:25486201; http://dx.doi.org/10.4161/15384101.2014.943567
- St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, et al. NT5E mutations and arterial calcifications. N Engl J Med 2011; 364:432-42; PMID:21288095; http://dx.doi.org/10.1056/NEJMoa0912923
- Markello TC, Pak LK, St Hilaire C, Dorward H, Ziegler SG, Chen MY, Chaganti K, Nussbaum RL, Boehm M, Gahl WA. Vascular pathology of medial arterial calcifications in NT5E deficiency: Implications for the role of adenosine in pseudoxanthoma elasticum. Mol Genet Metab 2011; 103:44-50; PMID:21371928; http://dx.doi.org/10.1016/j.ymgme.2011.01.018
- Renie WA, Pyeritz RE, Combs J, Fine SL. Pseudoxanthoma elasticum: high calcium intake in early life correlates with severity. Am J Med Genet 1984; 19:235-44; PMID:6507474; http://dx.doi.org/10.1002/ajmg.1320190205
- Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol 1988; 6:1-159; PMID:3359381; http://dx.doi.org/10.1016/0738-081X(88)90003-X
- LaRusso J, Li Q, Jiang Q, Uitto J. Elevated dietary magnesium prevents connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6−/−). J Invest Dermatol 2009; 129:1388-94; PMID:19122649; http://dx.doi.org/10.1038/jid.2008.391
- Li Q, LaRusso J, Grand-Pierre AE, Uitto J. Magnesium carbonate-containing phosphate binder prevents connective tissue mineralization in Abcc6−/− mice-potential for treatment of pseudoxanthoma elasticum. Clin Transl Sci 2009; 2:398-404; PMID:20443931; http://dx.doi.org/10.1111/j.1752-8062.2009.00161.x
- Jiang Q, Uitto J. Restricting dietary magnesium accelerates ectopic connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6−/−). Exp Dermatol 2012; 21:694-9; PMID:22897576; http://dx.doi.org/10.1111/j.1600-0625.2012.01553.x
- Li Q, Guo H, Chou DW, Berndt A, Sundberg JP and Uitto J. Mutant Enpp1asj mouse as a model for generalized arterial calcification of infancy. Dis Model Mech 2013; 6:1227-35; PMID:23798568; http://dx.doi.org/10.1242/dmm.012765
- Li Q, Pratt CH, Dionne LA, Fairfield H, Karst SY, Sundberg JP, Uitto J. Spontaneous asj-2 J mutant mouse as a model for generalized arterial calcification of infancy: A large deletion/insertion mutation in the Enpp1 gene. PLoS One 2014; 9:e113542; PMID:25479107; http://dx.doi.org/10.1371/journal.pone.0113542
- Berndt A, Li Q, Potter CS, Liang Y, Silva KA, Kennedy V, Uitto J, Sundberg JP. A single-nucleotide polymorphism in the Abcc6 gene associates with connective tissue mineralization in mice similar to targeted models for pseudoxanthoma elasticum. J Invest Dermatol 2013; 133:833-6; PMID:23014343; http://dx.doi.org/10.1038/jid.2012.340
- Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, Li K, Pulkkinen L, Birk DE, Sundberg JP, et al. Targeted ablation of the Abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol 2005; 25:8299-310; PMID:16135817; http://dx.doi.org/10.1128/MCB.25.18.8299-8310.2005
- Jiang Q, Li Q, Uitto J. Aberrant mineralization of connective tissues in a mouse model of pseudoxanthoma elasticum: Systemic and local regulatory factors. J Invest Dermatol 2007; 127:1392-402; PMID:17273159; http://dx.doi.org/10.1038/sj.jid.5700729
- NIH. Strengthening knowledge and understanding of dietary supplements. 2015. Available at: http://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
- Sherer DW, Singer G, Uribarri J, Phelps RG, Sapadin AN, Freund KB, Yanuzzi L, Fuchs W, Lebwohl M. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol 2005; 53:610-5; PMID:16198780; http://dx.doi.org/10.1016/j.jaad.2004.11.066
- Yoo JY, Blum RR, Singer GK, Stern DK, Emanuel PO, Fuchs W, Phelps RG, Terry SF, Lebwohl MG. A randomized controlled trial of oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol 2011; 65:341-8; PMID:21496949; http://dx.doi.org/10.1016/j.jaad.2010.05.023
- Li Q, Sundberg JP, Levine MA, Terry SF and Uitto J. The effects of bisphosphonates on ectopic soft tissue mineralization caused by mutations in the ABCC6 gene. Cell Cycle 2015; 14:1082-9; PMID:25607347; http://dx.doi.org/10.1080/15384101.2015.1007809
