DNA Topoisomerase IIα (Topo IIα) is essential for chromosome segregation in eukaryotes because it resolves catenations between the sister chromatid DNA molecules, thus allowing their separation in anaphase. It is the catalytic core of Topo II that performs this ancient topological transformation via a Strand Passage Reaction (SPR) in which one double helix is passed through another (see ). Curiously, the C-Terminal Domain (CTD) of Topo IIα is dispensable for the SPR but is needed for successful chromosome segregation. It recently became evident that the CTD provides a second crucial function of Topo II, as a protein-protein binding interface. In this issue of Cell Cycle, Ryu et al. provide compelling evidence that SUMOylation of the CTD triggers recruitment of a key cell division factor to mitotic kinetochores.Citation1
Figure 1. Dual Functions of Topo II. Top: Strand Passage Reaction: (1) G-segment DNA binds at catalytic core, (2) T-segment DNA captured by N-gate, (3) G-segment cleavage and T-segment passage, (4) G-segment re-ligation and T-segment release. Middle: Domain structure (colors match domains above). C-Terminal Domain (CTD) has not been crystalized, but emanates from the lowest portion of the C-gate. Bottom: CTD functions in protein recruitment to chromatin and kinetochores via SUMOylated (S) and phosphorylated (P) residues.
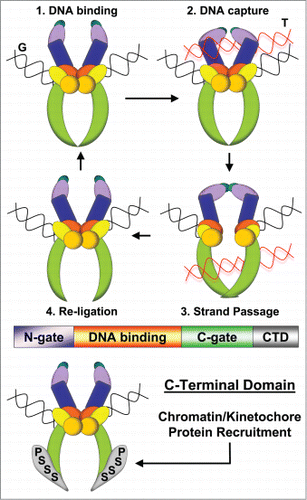
The SPR of Type II topoisomerases, including Topo IIα in eukaryotes, is required for life in all phyla. Not surprisingly these enzymes are highly conserved, albeit more so in the catalytic core than in the CTD where functional roles have not been well defined. The CTD is nevertheless subject to SUMOylation and phosphorylation at conserved residues and biological consequences of these post-translational modifications have begun to emerge. This has revealed that the CTD constitutes a second functional component of the enzyme.Citation1-5
In Ryu et al., the Azuma lab identified 3 conserved SUMOylation sites in the CTD of Xenopus Topo IIα.Citation1 Whether or not these sites were SUMOylated made no difference to the efficiency of the SPR. However, powerful biochemical approaches revealed the identity of mitotic chromatin proteins that specifically associate with the SUMOylated CTD in Xenopus Egg Extract (XEE). In particular, Claspin was identified and was shown to associate with the SUMOylated CTD dependent on its SIMs (SUMO interaction motifs). Claspin does not seem to be a promiscuous SUMO-binding protein, however, because it did not interact with other SUMOylated proteins. In mitosis, they observed for the first time that Claspin is abundant at kinetochores where it co-localizes with SUMO foci. This targeting was inhibited in the absence of SUMOylation. Because Topo IIα is enriched at kinetochores, and is likely the most abundant SUMO-modified protein at kinetochores in XEE, this strongly suggests that Topo IIα serves as a scaffold to directly recruit Claspin. This defines a novel function of the CTD that could explain its crucial role in mitosis.
These studies raise an important question: what is the kinetochore function of Claspin? Studies in other organisms have revealed functions of Claspin in DNA replication and in checkpoint signaling in response to stalled replication.Citation6 It is not clear if these roles contribute to mitosis. However, Claspin was recently implicated in activation of Aurora B,Citation7 and together with its kinetochore localization this places Claspin as a putative component of the error correction mechanism that ensures microtubule-kinetochore interactions produce stable bioriented chromosomes on the metaphase plate. Claspin may therefore be involved in mitotic regulation and perhaps mitotic checkpoints.
Intriguingly, the DNA damage checkpoint protein MDC1 binds to the CTD of human Topo IIα.Citation3 This interaction was induced by a catalytic inhibitor of Topo IIα that results in both SUMOylation and phosphorylation of the CTD. The data therefore implicate CTD post-translational modifications in activating checkpoint signals upon arrest of the SPR enzyme cycle. Subsequent studies in yeast used defined Topo IIα mutations to arrest the SPR, again resulting in CTD-dependent checkpoint activation.Citation4 The new insight from Ryu et al. synergize with these studies to reveal a compelling case for a novel role of the CTD as a checkpoint signaling hub that regulates progression through mitosis. It is clear that disturbances in the SPR of Topo IIα activate the CTD for checkpoint responses. What will be exciting to learn in future studies is whether the CTD plays roles in other signaling contexts to ensure faithful genome transmission in mitosis. Toward this goal it will be important to perform a comprehensive analysis of SUMOylation-dependent CTD binding proteins.
References
- Ryu H, et al. Cell Cycle 2015; 14(17):2777-84; http://dx.doi.org/10.1080/15384101.2015.1066537
- Bachant J, et al. Mol Cell 2002; 9: 1169-1182; PMID:12086615; http://dx.doi.org/10.1016/S1097-2765(02)00543-9
- Luo K, et al. Nat Cell Biol 2009; 11: 204-210; PMID:19098900; http://dx.doi.org/10.1038/ncb1828
- Furniss KL, et al. PLoS Genetics 2013; 9: e1003832; PMID:24098144; http://dx.doi.org/10.1371/journal.pgen.1003832
- Lane AB, et al. J Cell Biol 2013; 203: 471-486; PMID:24217621; http://dx.doi.org/10.1083/jcb.201303045
- Freire R, et al. Cell Cycle 2006; 5: 2831-2834; PMID:17172868
- Petsalaki E, et al. J Cell Sci 2013; 126: 1235-1246; PMID:23321637; http://dx.doi.org/10.1242/jcs.119677
