Lumenogenesis is a morphogenetic process by which organs generate an internal cavity. The function of lumens is vital and can vary from excretion, signaling, nutrition, mechanosensation or transport. Accordingly, defects in lumen formation cause a wide array of developmental diseases in humans, including spina bifida and polycystic kidney disease.
In recent years, some of the molecular mechanisms and cellular rearrangements participating in lumen initiation have been analyzed both in cultured cells and during in vivo organogenesis.Citation1 However, how lumens regulate their growth and the acquisition of a particular shape still remain elusive. In the Drosophila tracheas, anisotropic mechanisms for lumen growth have been identified. Those, lead to lumens that recapitulate the tubular shape of the organ.Citation2,3 Nonetheless, some lumens as the ones forming the heart or brain ventricles have more complex shapes. The understanding of these shaping mechanisms in vivo is challenging, as most of these organs are large and located deep in the embryo, hindering their in vivo imaging and whole 3D lumen reconstruction. We recently tackled this issue by imaging in real time the dynamics of lumenogenesis in the zebrafish inner ear,Citation4 a small and rather superficially located organ. Not studied before, the zebrafish otic vesicle lumen displays an asymmetric shape that resembles a biaxial ellipsoid, with one long axis and 2 equally sized short axes (). We uncovered that this asymmetry is achieved by the spatiotemporal regulation of various morphogenetic mechanisms.
Figure 1. Before the lumen forms, apical actomyosin accumulates in the midline along the long axis of the otic primordium. After expansion, the lumen approaches a bi-axial ellipsoidal shape. The rectangle highlights a region of the vesicle shown in the right scheme. In this square, an elongated cell in interphase changes its shape, decreasing the apicobasal length in order to round. This event pulls the epithelial surfaces (arrows) contracting the tissue. The dashed lines highlight the premitotic position of the apical (ap) and basal (ba) surfaces.
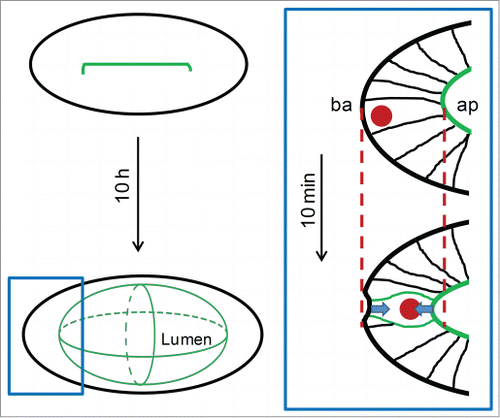
Cell proliferation played a relevant role in lumen shape both at early and late stages. Before the lumen forms, the number of cells that are assembled the otic primordium predetermines the gross length that the long axis of the lumen will have. Thus, when cell proliferation was abrogated during the early period, the inner ear lumen became a sphere instead of an ellipsoid. This seems to be due to the amount of cells contributing with apical membrane to the lumen (), similar to the extension of mammalian tubular organs.Citation1 The role of proliferation at later stages is much more unexpected and could only be revealed through a combination of high resolution imaging of mitotic events inside the epithelium and laser microsurgery experiments. We found that when a mitotic cell rounds immersed in an epithelium causes a mechanical deformation due to the cell attachment with their neighbors by apical junctions and basal adhesions. Mitotic rounding drives a local contraction of the epithelium by pulling the epithelial surfaces (, square), as a consequence of the reduction in length that the cell performs in order to round. These forces applied over the epithelium would rely on the increased cortical tension of the rounding cell as actomyosin accumulates in the cortex. In agreement with our observations, it was proposed that mitotic rounding cells in culture can exert forces against external objects.Citation5 Our recent experiments provide the first demonstration that this process occurs also in vivo within a packed epithelium. Moreover, we showed the relevance of the mitotic forces for lumen growth by inhibiting the cell cycle at different phases. This analysis indicated that cytokinesis is not essential for the contribution of proliferating cells to lumen growth in this time frame, and supports a central role for mitotic rounding. The mechanic role of mitotic cell rounding in morphogenesis is not restricted to lumen formation, as tracheal Drosophila invagination is also accelerated by tension released by rounding cells.Citation6 However, in the zebrafish otic primordium, individual rounding cells are not releasing tension in the tissue but instead exerting direct pulling forces.
Deformation and extension of the luminal surface is coordinated with fluid ingression into the cavity. From studies in other organs, the most accepted view of lumen growth by fluid entry proposes that the epithelium works as a pump that moves ions and water from outside the organ into the lumen. While this is also happening in the inner ear, we found that every epithelial cell also reduces its volume and changes its shape, thinning the epithelium.Citation4 This would result in a net redistribution of fluid from the cells to the lumen, a novel kind of fluid flow. This seems to be a general feature of lumen formation processes, as tissue thinning during lumen growth can be observed in many lumen-forming organs.Citation4
Finally, as both the tissue thinning and contraction are not occurring in a uniform manner over the entire vesicle, they constitute direct shaping mechanisms.Citation4 While the thinning occurs preferentially along the short axes, the contraction occurs close to the long axis poles. Thus, the anisotropic shaping mechanisms permit to build a lumen with particular shape, that although influenced by, does not completely recapitulate the shape of the organ (), as happens with tubes. This otic lumen will be then extensively remodeled to give rise to the lumen of the larval inner ear that includes functional hearing units, and in which the fluid of the lumen is essential for the mechanotrasduction properties of the organ. Thus, our analysis could help not only to improve our understanding of some highly frequent human hearing birth defects,Citation7 but also to contribute the knowledge of how fluids and forces sculpt cavities in vivo.
References
- Andrew DJ, Ewald AJ. Dev Biol 2010. 341:34-55. PMID:19778532; http://dx.doi.org/10.1016/j.ydbio.2009.09.024
- Förster D, Luschnig S. Nat Cell Biol 2012; 14:526-34. PMID:22446736; http://dx.doi.org/10.1038/ncb2456
- Nelson KS, et al. Nat Cell Biol 2012; 14:518-25. PMID:22446737; http://dx.doi.org/10.1038/ncb2467
- Hoijman E, et al. Nat commun 2015; 6:7355. PMID:26077034; http://dx.doi.org/10.1038/ncomms8355
- Stewart MP, et al. Nature 2011; 469:226-30. PMID:21196934; http://dx.doi.org/10.1038/nature09642
- Kondo T, Hayashi S. Nature 2013; 494:125-9. PMID:23334416; http://dx.doi.org/10.1038/nature11792
- Morton CC, Nance WE. N Engl J Med 2006; 354:2151-64. PMID:16707752; http://dx.doi.org/10.1056/NEJMra050700
