Cap-Dependent Translation and The Cell Cycle
Cap-dependent translation is a form of epigenetic regulation that is largely invisible to conventional gene expression analyses such as RNA seq and cDNA microarray. mTOR-dysregulated cap-dependent translation, however, constitutes one of the central features of a cancer cell and protein synthesis regulation has become an increasing focus of interest to cancer biologists. Are all capped mRNAs translated equally or is there an unrecognized layer of regulation at the level of protein translation? It is no longer assumed that once an mRNA is made, it will be predictably translated into a protein. A newly-discovered human cancer virus oncogene reveals cell cycle-dependent capped translation that provides an unexpected level of complexity for protein synthesis.
Protein translation has been long assumed to be the work of interphase cells based on total protein synthesis experiments dating back to the 1960s.Citation1 Interphase restricted capped protein translation is ascribed to the loss of a cellular mRNA pool during G2/M chromatin condensation and to mitotic activation of a key negative translation regulator, eIF4E-binding protein 1 (4E-BP1). 4E-BP1 is phosphorylated by mTOR,Citation2 resulting in its inhibition, therefore, reduced mTOR activity during mitosis should lead to activation of this gate-keeper and decreased cap-dependent translation.
Actual measurements of mitosis-associated cap-dependent translation (MACT), however, are technically challenging. Mitosis is relatively short (˜45 mins to 2 hours) and mitotic synchronization agents such as nocodazole that inhibit microtubule polymerization also inhibit cap-dependent protein synthesis.Citation3,4 Coldwell et al. recently showed, using alternative cell synchronization methods, that cap-dependent translation actually continues or even increases during mitosis.Citation3
During interphase, cap-dependent protein translation is initiated by the eIF4F cap initiation complex composed of the cap (m7GpppN)-binding protein eIF4E, the RNA helicase eIF4A, and a scaffolding protein eIF4G. 4E-BP1 sequesters eIF4E from the cap initiation complex but when 4E-BP1 is phosphorylated by mTOR kinase, eIF4E is released to facilitate cap-dependent translation.Citation5 4E-BP1 is phosphorylated by mTOR at multiple sites that can be distinguished by SDS-PAGE analysis into at least 4 distinct 4E-BP1 phosphospecies, named α through δ (lowest to highest phosphorylation form) according to molecular mass. Interphase activation of mTOR, a nutrient sensor regulated by amino acid levels and PI3K-Akt signaling, is required for the translation of proteins needed during cytokinesis.
Mitosis-Associated Cap-Dependent Translation (MACT)
A rare human skin cancer gives clues on how protein synthesis is regulated during mitosis. Most Merkel cell carcinomas are caused by a newly-discovered human polyomavirus (Merkel cell polyomavirus, MCV) found by digital transcriptome subtraction.Citation6 The MCV small T (sT) oncoprotein targets cellular E3 ligases, including cdc20, promoting mitotic entry and activation of mitotic kinases such as CDK1/CYCB1.Citation4
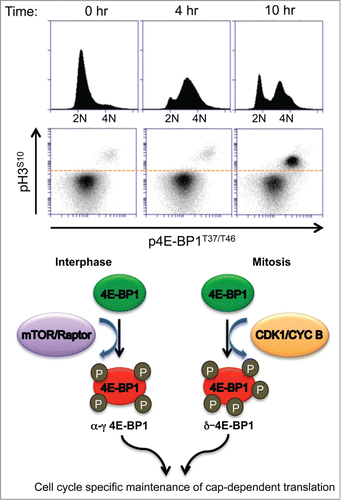
In addition to its other typical mitotic targets, CDK1 phosphorylates and inactivates 4E-BP1. Although CDK1 can substitute for mTOR in phosphorylating 4E-BP1 at canonical sites, resulting in release of eIF4E, it also phosphorylates 4E-BP1 at novel sites to form the mitosis-specific δ-4E-BP1 isoform.Citation4 This is not an insignificant activity. As cells enter mitosis, levels of 4E-BP1 phosphorylation are higher than in any other part of the cell cycle (). The differences in activity between mitotic and non-mitotic 4E-BP1 isoforms are unknown but structural studies suggest that these differences might alter 4E-BP1-eIF4E affinities, providing a potential discriminatory mechanism for translation of different classes of capped mRNAs. Importantly, interphase 4E-BP1 inactivation is sensitive to mTOR inhibitors, such as Torin1 and PP242, whereas mitotic 4E-BP1 inactivation is not. While 4E-BP1 hyperphosphorylation is commonly assumed to simply inhibit 4E-BP1’s gatekeeper activity, there is growing evidence that the δ-4E-BP1 may have a gain-of-function that is critical for successful mitogenesis.
Figure 1. CDK1 in mitotic cells hyperphosphorylates 4E-BP1, the key checkpoint for cap-dependent translation. This figure shows increasing 4E-BP1 phosphorylation in double-thymidine arrested cells at time 0 and are synchronized to maximally enter mitosis at 10 hours.
MCV sT directly transforms rodent cells and induces the mTOR inhibitor-resistant δ-4E-PB1 isoform.Citation7 sT-induced cell transformation can be reversed by expression of a dominant-positive form of 4E-BP1 having alanine substitutions at key priming phosphorylation residues so that it is constitutively active and no longer regulated by mTOR or CDK1. These results point toward the possibility that mitotic proteins expressed through cap-dependent translation might be contributing to cancer cell proliferation.
Although 4E-BP1 is inactivated during mitosis, downstream regulatory checkpoints of the translational machinery are still critical in determining whether or not translation occurs. We examined a series of cell lines for mitotic protein synthesis using a fluorescent methionine analogue. Using flow cytometry, nascent protein synthesis could be directly measured in cells positive (mitotic) or negative (interphase) for the mitosis marker, phospho-ser10 histone H3, thus avoiding the use of nocodazole. New small molecule inhibitors of the cap-initiation complex allow discrimination between cap-dependent and -independent protein synthesis. Contrary to dogma, we find that new protein synthesis is comparable between mitosis and interphase for most, but not all cell lines, and that the majority of mitotic protein synthesis is cap-dependent and relies on phosphorylation levels of 4E-BP1. For BJ-tert human fibroblast cells expressing MCV sT, MACT is resistant to mTOR inhibitors, consistent with a CDK1-mediated regulatory mechanism.
The reasons why mTOR hands off its protein-regulatory activity to CDK1 during mitosis are not clear. Activated cap-dependent translation of pre-existing mRNAs provides the most rapid means for gene expression, which is likely to be critical during the short time-scale of mitosis. Since mitotic 4E-BP1 phosphorylation by CDK1 is distinct from interphase mTOR phosphorylation patterns, the potential for nuances in the control of cap-dependent translation exists that may lead to differential protein expression profiles during CDK1 regulation. If CDK1-regulated protein synthesis plays a role in cancer cell proliferation, combination therapies to block cap-dependent translation during both interphase and mitosis might be particularly effective.
References
- Prescott DM, Bender MA. Exp Cell Res 1962; 26:260–8; PMID:14488623; http://dx.doi.org/10.1016/0014-4827(62)90176-3
- Burnett PE, et al. Proc Natl Acad Sci USA 1998; 95:1432–7; PMID:9465032; http://dx.doi.org/10.1073/pnas.95.4.1432
- Coldwell MJ, et al. Cell Cycle 2013; 12:3615–28; PMID:24091728; http://dx.doi.org/10.4161/cc.26588
- Shuda M, et al. Proc Natl Acad Sci USA 2015; 112:5875–82; PMID:25883264; http://dx.doi.org/10.1073/pnas.1505787112
- Gingras AC, et al. Genes Development 2001; 15:807–26; PMID:11297505; http://dx.doi.org/10.1101/gad.887201
- Feng H, et al. Science 2008; 319:1096–100; PMID:18202256; http://dx.doi.org/10.1126/science.1152586
- Shuda M, et al. J Clinical Investigation 2011; 121:3623–34; PMID:21841310; http://dx.doi.org/10.1172/JCI46323
