Canonical epidermal growth factor receptor (EGFR) interaction with cognate ligands such as EGF triggers receptor dimerization, activation and a well-characterized endocytic trafficking pathway that leads to termination of signaling via lysosomal degradation of the receptor. We,Citation1 and others,Citation2,3 have investigated the existence of an alternative route of EGFR trafficking, that occurs as a cellular response to stress and in the absence of ligand. Following exposure to oxidative stress, triggered by a range of insults such as ultraviolet light C (UVC) or the chemotherapeutic agent cisplatin, EGFR is internalised in a p38 MAP kinase-dependent manner,Citation2,3 but, contrary to ligand-stimulated EGFR internalisation, this occurs in the absence of receptor activation or ubiquitination.Citation1 Post-internalisation, ligand-stimulated EGFR undergo ubiquitin-dependent interaction with the Endosomal Sorting Complex Required for Transport (ESCRT machinery) that sorts them onto intraluminal vesicles (ILVs) of multivesicular bodies (MVBs). This spatially segregates them from non-ubiquitinated receptors that remain on the MVB limiting membrane and undergo Rab11-dependent recycling to the plasma membrane. When all the recycling receptors have been removed, these MVBs fuse with lysosomes and the ILVs containing ligand-stimulated EGFR are degraded. In early endosomes, stress-exposed EGFR is sorted away from its ligand-stimulated counterpart into a distinct population of relatively stable MVBs where they accumulate on both the ILVs and the MVB limiting membrane. Segregation of ligand-stimulated and stress-induced EGFR in early endosomes is orchestrated by the WASH complex,Citation1 presumably involving its actin polymerisation-driven endosomal subdomain specification properties.Citation4 Analysis of exogenously-expressed potential cargoes that might accompany stress-exposed EGFR into this particular subtype of MVB lead us to conclude that these MVBs represent endosomal precursors to lysosome-related organelles, such as melanosomes. Their presence not only in specialized cell types such as melanocytes,Citation5 but, also, as shown in our study,Citation1 in non-specialized cells such as HeLa, suggests that they have additional functions.
Establishing the functional significance of the perinuclear MVBs in which stress-induced EGFR accumulates has been facilitated by our identification of molecular requirements regulating EGFR retention in these MVBs. Inhibition of p38 after accumulation of stress-induced EGFR in perinuclear MVBs caused the EGFR to reappear on the plasma membrane, demonstrating a second role for p38 in regulating stress-induced EGFR trafficking, distinct from its previously identified role in EGFR internalisation. Plasma membrane reappearance of EGFR that was previously sequestered within MVBs also indicates that EGFR-containing ILVs can back-fuse with the MVB limiting membrane to allow recycling. Retention of stress-induced EGFR in MVBs also involves the ESCRT machinery and the ESCRT-accessory protein, ALIX, which is necessary for the sorting of non-ubiquitinated EGFR onto ILVs.Citation1 P38/ESCRT/ALIX-dependent accumulation of EGFR in perinuclear MVBs is accompanied by slow, but sustained, EGFR activation and downstream signaling. Surprisingly, and contrary to receptor activity following ligand binding, EGFR activation does not occur if internalisation from the plasma membrane is abrogated.Citation1 As maintenance of signaling is enabled by the ALIX-driven sequestration of stress-exposed EGFR in MVBs,Citation1 we hypothesize that repeated rounds of ESCRT and ALIX-dependent internalisation and back-fusion of EGFR to and from ILVs ensures continued accessibility of the receptor to downstream signaling molecules. This hypothesis is reinforced by the previously suggested role of ALIX in ILV back-fusion events during nucleocapsid release to the cytosol from endosomes in the process of viral infection.Citation6
Signaling from EGFR in perinuclear MVBs delayed apoptosis induced by UVC and cisplatin, suggesting that sequestration of EGFR in this subtype of MVB could contribute to the acquisition of resistance to chemotherapy. This highlights the importance of determining the trafficking response of EGFR in tumors in response to therapy. This is of particular relevance to the design of combination therapies that utilize chemotherapeutic agents in combination with anti-EGFR therapies.
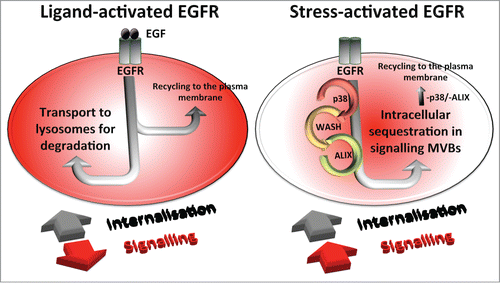
The study of the atypical pathway of stress-triggered EGFR trafficking and its comparison with the better-characterized ligand-stimulated one (as schematized in Fig. 1) has extended our knowledge of the variety of mechanisms regulating EGFR trafficking along the endocytic pathway. It has also highlighted the multiplicity of destinations for this, and presumably other signaling receptors and the pivotal effects of the different trafficking outcomes on signaling. Moreover, our study reveals the existence of at least 2 coexisting subpopulations of MVBs, which, in parallel to the previously identified different subpopulations of ILVs within the same MVB,Citation7 highlights the complexity associated with this trafficking organelle and its key role in regulating receptor signaling in mammalian cells.
Figure 1. Ligand-bound, ubiquitinated EGFR are activated and signal at the plasma membrane. Post-internalisation they undergo ESCRT-mediated sorting to ILVs of MVBs that separates them from non-ubiquitinated recycling receptors and promotes their lysosomal degradation. Stress-induced non-ubiquitinated EGFR are activated post-internalisation and sequestered on ILVs of a separate subtype of signaling MVBs by the action of p38, WASH complex and ESCRT/ALIX.
References
- Tomas A, et al. Nat Commun 2015; 6:7324; PMID:26066081; http://dx.doi.org/10.1038/ncomms8324
- Zwang Y, Yarden Y. EMBO J 2006; 25:4195-4206; PMID:16932740; http://dx.doi.org/10.1038/sj.emboj.7601297
- Grandal MV, et al. Traffic 2012; 13:576-585; PMID:22192528
- Bear JE. Dev Cell 2009; 17:583-584; PMID:19922862; http://dx.doi.org/10.1016/j.devcel.2009.10.019
- van Niel G, et al. Dev Cell 2011; 21:708-21; PMID:21962903; http://dx.doi.org/10.1016/j.devcel.2011.08.019
- Bissig C, et al. Dev Cell 2013; 25:364-73; PMID:23664863; http://dx.doi.org/10.1016/j.devcel.2013.04.003
- Edgar JR, et al. Traffic 2014; 15:197-211; PMID:24279430; http://dx.doi.org/10.1111/tra.12139
