Paracrine and juxtacrine interactions between cancer cells and the tumor microenvironment have been shown to be critical determinants of tumor initiation and progression. Extensive efforts are being made to identify these reciprocal interactions mediated by cytokines, chemokines and growth factors.Citation1 Earlier we have shown that the co-grafting of heterogeneous small cell lung cancer (SCLC) subclones, one growing in suspension with neuroendocrine features (NE cell) and the other clone with adherent mesenchymal-like features (NonNE cell) promotes metastasis whereas single clones of either NE or NonNE cells alone only results in local growth.Citation2 Our data show that tumor heterogeneity can have functional significance resulting in the co-evolution of tumor subclones thereby creating a tumor microenvironment from which NE tumor cells can more effectively metastasize. The inherent genetic instability of tumor subclones might make it easier to select for rare variants that support such crosstalk thereby creating a symbiotic relationship. In this way the formation of a “tumor organ” reflects an evolutionary process in which differentiation of tasks by tumor subclones becomes an important feature.
Clonal cooperation in tumor progression has been described for mammary tumorigenesis in which 2 types of clones cooperate to form mammary tumors by paracrine interactions whereas neither of the clones can drive tumor formation by itself.Citation3 Similarly, we have shown that mixed NE and NonNE cell populations gain synergistic proliferation benefits in in vitro cell proliferating assay.Citation2 This suggests that a close vicinity of NE and NonNE cells promotes rapid tumor growth. However, subcutaneously grafting of mixed populations did not show accelerated local tumor growth as compared to the injection of a single clone population.Citation2 This discrepancy might simply be caused by the fact that the subcutaneous microenvironment is more permissive for growth of this tumor. In line with this, we have shown that intravenous grafting of neuroendocrine SCLC clones does result in colonization of liver rather than lung even though many of the tumor cells are initially trapped in lung.Citation4 Although IV injected NE cells colonized the liver with similar efficiency as NE and NonNE mixtures colonization of mediastinal brown fat was clearly enhanced by IV co-injection of NonNE cells. We also noted a single tumor in lung after IV injection of the mixture. This tumor carried both cell types suggesting that NonNE cells might be required for colonization of some but not other tissues ().Citation4 Therefore, the nature of the local cell-cell contacts and the specific microenvironmental conditions in lung likely promote the co-selection of NE and NonNE clones to facilitate local tumor growth while at the same time facilitating metastatic spread of the NE tumor component.
Figure 1. Intravenous injection of neuroendocrine (NE) cell alone generates liver metastasis, while co-injection of NE and mesenchymal-like (NonNE) tumor cells into the bloodstream also causes frequent mediastinal metastasis and occasional lung metastasis. NonNE tumor cells in subcutaneously transplanted mice are crucial for the invasion capacity of NE tumor cells (Top). Fgf2 secreted by NonNE cells induces Pea3 expression via MAPK activation in NE cells. Pea3 induction is required for the metastasis of NE cells. NonNE cells also secrete extracellular matrix (ECM) remodeling factors such as LOX, Cathepsin, and PAI-1. These ECM modifiers likely further facilitate metastasis of SCLC (Bottom).
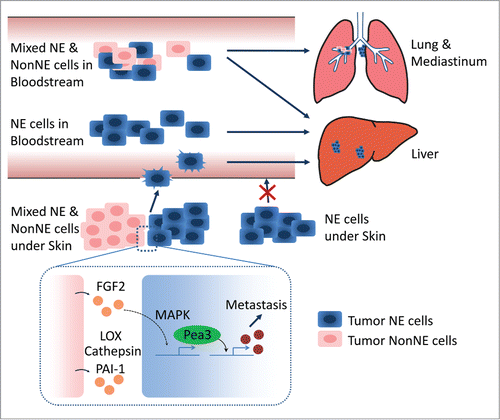
In the current study we have also focused on the factors and signaling pathways that enable NE cells to metastasize. Since metastasis of NE tumor cells from subcutaneous grafts to liver requires co-grafting of NonNE subclones while the metastasized tumors were exclusively composed of NE cells we reasoned that the interaction between the NonNE and NE tumors cells should elicit a change in expression in NE cells that potentiates their capacity to become locally invasive thereby facilitating their entry into the circulation. Analysis of the changes in expression pattern in NE tumor cells when either grown as pure population or as mixtures, showed that upregulation of Pea3 was the most pronounced in NE tumor cells. Interestingly, Pea3 is known to play a critical role in tumor invasiveness and metastasis and its expression level has been correlated with metastatic behavior and overall survival of malignant tumors.Citation5 In in vitro invasion assays and in vivo subcutaneous graft experiments, the expression of Pea3 appeared essential for the invasion activity and metastatic capacity of NE cells as its downregulation abrogated metastatic spread without influencing tumor growth at the subcutaneous inoculation site. Overexpression of Pea3 in NE cells endowed them with invasion activity through matrigel in the absence of conditioned medium from NonNE cells. However, Pea3 overexpressing NE cells conferred only partial metastatic capacity indicating that Pea3 is not sufficient to fully substitute for the factors contributed by NonNE cells. Interestingly, LC-MS/MS secretome analysis identified that NonNE cells secret many extracellular matrix (ECM) remodeling factors such as Protein-lysine 6-oxidase (LOX), Cathepsin, and Plasminogen activator inhibitor 1 (MC, Kwon et al., unpublished data). Therefore, Pea3 overexpressing NE cells might need these ECM modifiers for their metastatic spread from subcutaneous primary tumors. We further found that Fgf2 secreted by NonNE cells induces the expression of Pea3 via MAPK signaling activation and decreased expression of Fgf2 in NonNE cells reduced the expression of Pea3 with concomitant impaired invasion activity of NE cells (). The notion that NE cells that have metastasized have not acquired autonomous metastatic capacity, and upon isolation again require the co-grafting with NonNE cells to metastasize from subcutaneous sites further points to the significance of the paracrine interaction established between NonNE and NE tumor subclones. This appears a recurrent theme in the SCLC mouse model as similar cell populations with the same paracrine interactions could be isolated from multiple independent tumors.
References
- Junttila MR, de Sauvage FJ. Nature 2013; 501:346-54; PMID:24048067; http://dx.doi.org/10.1038/nature12626
- Calbo J, et al. Cancer Cell 2011; 19:244-56; PMID:21316603; http://dx.doi.org/10.1016/j.ccr.2010.12.021
- Cleary AS, et al. Nature 2014; 508:113-7; PMID:24695311; http://dx.doi.org/10.1038/nature13187
- Kwon MC, et al. Genes Dev 2015; 29:1587-92; PMID:26215568; http://dx.doi.org/10.1101/gad.262998.115
- Oh S, et al. Biochimica Et biophysica Acta 2012; 1826:1-12; PMID:22425584
