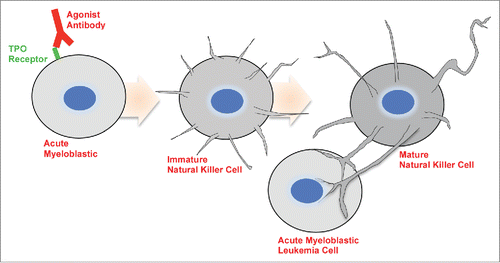
Recently we reported on the selection of an antibody that induces human acute myeloblastic leukemic (AML) cells to transform into natural killer (NK) cells.Citation1 The overall process involves selection of an antibody that transforms AML cells into NK cells that then kill their erstwhile brothers or sisters in a fratricidal process (). These induced NK cells produce almost all of the cytotoxic molecules associated with NK cells including perforin, granzyme B, and Interferon γ. The induced NK cells appear to deliver the cytoxic molecules via long filopodia that appear to make holes in the target cell plasma membrane and enter the cytoplasm of the target cells thru these holes-much like a gasoline pump.
Figure 1. When grown for short periods of time with antibody to the TPO receptor, the leukemia cells are induced to form round cells with “needle like” filopodia. When grown for longer periods, the induced cells present more elongated-dendrites. Some of the projections from the induced cells interact with the target cells and lyse them by injecting cellular toxins.
This most recent observation is, in reality, only the latest finding concerning a series of antibodies that operate by a process we call “receptor pleiotropism” where antibodies induce cell fates that are very different than those induced by the natural agonist to the same receptor.Citation2-4 Thus, the logic of the experiment was that since agonist antibodies to known receptors induce alternative fates in normal stem cells why not try the same in malignant cells that often are very stem cell like. The advantage here is that tumor cells could be converted to a less malignant phenotype. The induction of killer cells is simply a bonus to the overall objective. The main question concerns why antibody agonists induce cell fates that can be so different than those induced by the natural agonist to the same receptor. To explain, one starts with the notion that signal transduction (ST) pathways can be highly degenerate. Thus, natural agonists must be highly tuned by evolution to guide the cell through a perfectly timed set of contingent events. And since initiation of the cascade most often begins at the cell surface we must look there for an explanation. In a chemical sense there are not many ways that events at the cell surface can influence downstream ST events other than control of binding energy whose on and off rates regulate the sustainability of a signal of induction or a conformational change in the receptor. Also, receptor internalization is, to first approximation, the equivalent of increasing the off rate of an agonist. Thus, antibodies with their high binding energy can lead to more sustained signaling. The viability of such a hypothesis relies on the likely premise that cells “read” not only the absence or presence of ST factors but also their kinetics and overall concentrations. These kinetic factors are likely to be very different between antibodies and the natural agonist even though they both bind to the same receptor. Indeed, in the recent paper where thrombopoietin receptor is the activated receptor, there is no evidence for a change in the activation of ST factors, but there is a large change in the kinetics of their appearance and disappearance.
Aside from their usefulness in the regulation of cell fates, orthogonal agonists such as antibodies can teach us much about the chemistry and biochemistry of the combinatorial matrix of signal transduction factors. Because we have a comparator, we can learn how the kinetics and concentrations of molecules control cell fates. In the end it may be as much about the disappearance of molecules as it is their appearance. For example, we know from classical chemical studies of pathways such as glycolysis that the Kms of enzymes that control a pathway are highly tuned to the expected concentrations of substrates that appear as the pathway proceeds. We should expect the same for ST pathways except that because of the degeneracy of members of the cascade alteration of chemical parameters can lead to different cell fates. Thus, we know much about the characters in the ST “play” but less about their chemistry. The study of orthogonal agonists operating through receptor pleiotropism may help us fill in the gaps that are presently mostly chemical and include both thermodynamic and kinetic parameters.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Yea K, et al. Proc Natl Acad Sci USA 2015; 112(45):E6158-65 201519079; PMID:26487683; http://dx.doi.org/10.1073/pnas.1519079112
- Xie J, et al. Proc Natl Acad Sci USA 2013; 14; 110(20):8099-104; PMID:23613575; http://dx.doi.org/10.1073/pnas.1306263110
- Yea K, et al. Curr Opin Chem Biol 2015; 26:1-7; PMID:25621729; http://dx.doi.org/10.1016/j.cbpa.2015.01.002
- Yea K, et al. Proc Natl Acad Sci USA 2013; 10; 110(37):14966-71; PMID:23980154; http://dx.doi.org/10.1073/pnas.1313671110
