ABSTRACT
Expression of Breast Cancer Metastasis Suppressor 1 (BRMS1) reduces the incidence of metastasis in many human cancers, without affecting tumorigenesis. BRMS1 carries out this function through several mechanisms, including regulation of gene expression by binding to the mSin3/histone deacetylase (HDAC) transcriptional repressor complex. In the present study, we show that BRMS1 is a novel substrate of Cyclin-Dependent Kinase 2 (CDK2) that is phosphorylated on serine 237 (S237). Although CDKs are known to regulate cell cycle progression, the mutation of BRMS1 on serine 237 did not affect cell cycle progression and proliferation of MDA-MB-231 breast cancer cells; however, their migration was affected. Phosphorylation of BRMS1 does not affect its association with the mSin3/HDAC transcriptional repressor complex or its transcriptional repressor activity. The serine 237 phosphorylation site is immediately proximal to a C-terminal nuclear localization sequence that plays an important role in BRMS1-mediated metastasis suppression but phosphorylation does not control BRMS1 subcellular localization. Our studies demonstrate that CDK-mediated phosphorylation of BRMS1 regulates the migration of tumor cells.
Keywords:
Introduction
The cell cycle is a highly conserved process that leads to cell division in all organisms, from simple prokaryotes to highly evolved eukaryotes. Due to its crucial role in the fundamental basis of growth, the cell cycle is a tightly regulated, responding only to stimulatory and developmental cues, such as mitogenic and growth factors.Citation1,2 The key enzymes involved in promoting cell cycle progression are the Cyclin-dependent kinases (CDKs), which are dimeric enzymes that consist of a protein kinase subunit that requires the binding of a regulatory Cyclin subunit for activation.Citation3 In eukaryotes, progression through the different cell cycle phases is regulated by distinct Cyclin/CDK complexes. For example, progression from G0 to G1 phase is mediated by Cyclin D/CDK4/6,Citation4,5 while entry from G1 into S phase is promoted by Cyclin E/CDK2.Citation6 Cyclin A/CDK2 is important during S phase, followed by the activity of Cyclin A/CDK1 in G2.Citation7 Finally, Cyclin B/CDK1 is the key Cyclin/CDK complex important in promoting mitosis.Citation8,9 The activity of different Cyclin/CDK complexes mediates phosphorylation of some overlapping but also distinct substrates necessary for progression during each phase of the cell cycle.Citation3
One key, well-characterized Cyclin/CDK substrate is the retinoblastoma protein (pRb). pRb is a tumor-suppressor protein, which controls progression from G1 to S phase of the cell cycle.Citation10,11 pRb binds to and inhibits the transcription factor E2F, whose activity is necessary for the transcription of genes required for S phase progression.Citation11-13 Mechanistically, pRb inhibits E2F activity through direct binding to its transactivation domainCitation14 and recruiting the mSin3/histone deacetylase (HDAC) complex.Citation15-17 HDACs mediate the deacetylation of histones at the E2F promoter, leading to more compact nucleosomes, thereby preventing access to transcriptional co-activators to inhibit transcription.Citation18 pRb recruitment of the mSin3/HDAC transcriptional repressor complex to E2F is mediated by retinoblastoma-binding protein 1 (RBP1).Citation19-21 RBP1 binds to the pocket region of pRb via an LXCXE consensus motif,Citation20 found on many pRb-binding partners.Citation22 RBP1 also binds to the mSin3-associated protein of 30-kDa (SAP30),Citation19 which in turn interacts with the mSin3 scaffold protein.Citation23 Together, SAP30 and mSin3 are part of a complex consisting of several subunits,Citation24 including SAP18,Citation25 SAP45,Citation26 retinoblastoma-associated proteins of 46/48kDa (RbAP46/48)Citation27,28 and HDAC1/2. Therefore, RBP1 acts as a bridging protein between pRb/E2F and the mSin3/HDAC transcriptional repressor complex.
The cumulative phosphorylation of pRb and RBP1 by Cyclin D/CDK4/6 and Cyclin E/CDK2Citation21,29-32 mediates the dissociation of these proteins and the mSin3/HDAC complex from E2F,Citation21,33-35 to abolish their inhibitory effect on E2F, allowing transcription to occur and progression from G1 into S phase.
Interestingly, in addition to binding the pRb/E2F complex and regulating cell cycle progression, RBP1 also interacts with transcriptional regulators with roles distinct from the cell cycle; this includes the metastasis suppressor protein, Breast Cancer Metastasis Suppressor 1 (BRMS1).Citation36 BRMS1 was identified by differential displayCitation37 and is a member of the family of metastasis suppressor proteins.Citation38 Metastasis suppressor proteins are important for inhibiting the spread of tumor cells, without affecting their tumorigenesis. BRMS1 metastasis suppressor function may be mediated by a number of mechanisms, including through transcriptional control of genes involved in metastasisCitation39-42 and through its association with the RBP1/mSin3/HDAC transcriptional repressor complex.
Since our previous studies have shown that RBP1 is a novel Cyclin/CDK substrate,Citation21 we investigated if Cyclin/CDKs may regulate the BRMS1 protein, since it is a binding partner of RBP1. We find that BRMS1 is a novel Cyclin/CDK substrate that is phosphorylated on serine 237. Consistent with our findings, recent reports show that BRMS1 serine 237 is phosphorylated in several different cell lines, such as KG1 acute myeloid leukemia cells,Citation43 human H1, H9 HUES9 and Odense-3 embryonic stem cells, DF19.7 pluripotent stem cells and NFF fibroblasts,Citation44,45 HeLa cervical cancer cellsCitation46,47 colorectal cancer cellsCitation48 and liver cells.Citation49 Our studies now show that while the phosphorylation of serine 237 on BRMS1 has no effect on cell cycle progression or proliferation, it does modulate the motility and therefore migration of MDA-MB-231 breast cancer cells. This regulation does not appear to be due to phosphorylation controlling transcriptional repression mediated by BRMS1. These findings provide insights into the regulation of BRMS1 by phosphorylation and its role as a metastasis suppressor.
Results
BRMS1 is a novel CDK substrate
Previous studies in our laboratory identified RBP1 as a novel CDK substrate that is phosphorylated in a cell-cycle dependent manner to regulate its association with the pRb tumor suppressor protein.Citation21 RBP1 has several binding partners,Citation19,20 including the metastasis suppressor BRMS1,Citation36 which prompted us to investigate if this protein may also be phosphorylated by CDKs to regulate its function. BRMS1 is a 246 amino acid protein, consisting of several domains including; multiple imperfect leucine zipper domains, 2 coiled-coil domains that are important for protein-protein interactions, and 2 nuclear localization signals (NLS) sequences ().Citation37 Sequence analysis of BRMS1 revealed that serine 237 lies between the 2 NLS sequences and conforms to a CDK consensus phosphorylation site, which consists of a phosphorylated serine or threonine followed by an obligatory C-terminal proline (S/T-P), with a positively charged arginine or lysine often present in the +3 positionCitation50,51 ().
Figure 1. BRMS1 is a novel CDK substrate. (A) Domain structure of BRMS1, which contains imperfect Leucine zippers, coiled-coil domains, nuclear localization sequences and a potential CDK phosphorylation site, located at serine 237. (B) BRMS1 is phosphorylated in cells by CDKs. Left panel, HEK-293T cells transfected with pCMV-Tag2A vector (Lane 1) or pCMV-Tag2A-BRMS1 (Lane 2 and 3), were 32P-labeled in the absence (Lanes 1 and 3) or presence (Lane 2) of 50 μM Roscotivine. Immunoprecipitated FLAG-tagged BRMS1 was separated by SDS-PAGE and transferred onto nitrocellulose. BRMS1 phosphorylation was detected by autoradiography (top panel). Western blotting was performed with an anti-FLAG antibody to demonstrate equal levels of BRMS1 in lanes 2 and 3 (bottom panel). Right panel: Phosphorylated BRMS1 purified from transfected HEK-293T cells was subjected to phosphoamino acid analysis. Phosphoamino acids were visualised by ninhydrin staining and autoradiography. Arrows indicate the positions of phosphoserine (P-Ser), phosphothreonine (P-Thr) and phoshotyrosine (P-Tyr). (C) In vitro phosphorylation of BRMS1 by Cyclin A/CDK2. Left panel: purified His6-tagged wild-type BRMS1 or BRMS1 S237A were incubated with or without Cyclin A/CDK2 in the presence of [γ-32P] ATP. Following phosphorylation, samples were separated on SDS-PAGE, stained with Coomassie brilliant blue (bottom panel) and autoradiographed (top panel). Right panel: purified His6-tagged BRMS1 phosphorylated in vitro by Cyclin A/CDK2 in the presence of [γ-32P] ATP was subjected to phosphoamino acid analysis. Phosphoamino acids were visualized by ninhydrin staining and autoradiography. Arrows indicate the positions of phosphoserine (P-Ser), phosphotheorine (P-Thr) and phosphotyrosine (P-Tyr). (D) BRMS1 is phosphorylated on Ser 237. In vitro or in vivo phosphorylated BRMS1 (from B and C) was separated by SDS-PAGE, excised and subjected to tryptic digestion. Phosphopeptides were then purified on TiO2 beads and analyzed by LC/MS. Tandem MS/MS mass spectra of phosphorylated serine 237 (pS237) from His6-BRMS1 phosphopeptide 234–241 (AAVpSPQKR), parent ion: 468.8 m/z 2+ (indicated with an arrow), following phosphorylation in vitro by CyclinA/CDK2 (top panel), or following immunoprecipitation of FLAG-tagged BRMS1 from BT-549 cells (bottom panel).
![Figure 1. BRMS1 is a novel CDK substrate. (A) Domain structure of BRMS1, which contains imperfect Leucine zippers, coiled-coil domains, nuclear localization sequences and a potential CDK phosphorylation site, located at serine 237. (B) BRMS1 is phosphorylated in cells by CDKs. Left panel, HEK-293T cells transfected with pCMV-Tag2A vector (Lane 1) or pCMV-Tag2A-BRMS1 (Lane 2 and 3), were 32P-labeled in the absence (Lanes 1 and 3) or presence (Lane 2) of 50 μM Roscotivine. Immunoprecipitated FLAG-tagged BRMS1 was separated by SDS-PAGE and transferred onto nitrocellulose. BRMS1 phosphorylation was detected by autoradiography (top panel). Western blotting was performed with an anti-FLAG antibody to demonstrate equal levels of BRMS1 in lanes 2 and 3 (bottom panel). Right panel: Phosphorylated BRMS1 purified from transfected HEK-293T cells was subjected to phosphoamino acid analysis. Phosphoamino acids were visualised by ninhydrin staining and autoradiography. Arrows indicate the positions of phosphoserine (P-Ser), phosphothreonine (P-Thr) and phoshotyrosine (P-Tyr). (C) In vitro phosphorylation of BRMS1 by Cyclin A/CDK2. Left panel: purified His6-tagged wild-type BRMS1 or BRMS1 S237A were incubated with or without Cyclin A/CDK2 in the presence of [γ-32P] ATP. Following phosphorylation, samples were separated on SDS-PAGE, stained with Coomassie brilliant blue (bottom panel) and autoradiographed (top panel). Right panel: purified His6-tagged BRMS1 phosphorylated in vitro by Cyclin A/CDK2 in the presence of [γ-32P] ATP was subjected to phosphoamino acid analysis. Phosphoamino acids were visualized by ninhydrin staining and autoradiography. Arrows indicate the positions of phosphoserine (P-Ser), phosphotheorine (P-Thr) and phosphotyrosine (P-Tyr). (D) BRMS1 is phosphorylated on Ser 237. In vitro or in vivo phosphorylated BRMS1 (from B and C) was separated by SDS-PAGE, excised and subjected to tryptic digestion. Phosphopeptides were then purified on TiO2 beads and analyzed by LC/MS. Tandem MS/MS mass spectra of phosphorylated serine 237 (pS237) from His6-BRMS1 phosphopeptide 234–241 (AAVpSPQKR), parent ion: 468.8 m/z 2+ (indicated with an arrow), following phosphorylation in vitro by CyclinA/CDK2 (top panel), or following immunoprecipitation of FLAG-tagged BRMS1 from BT-549 cells (bottom panel).](/cms/asset/80cc1bcb-5cc4-4f98-8c6c-3201eb9b15b0/kccy_a_1121328_f0001_oc.gif)
To investigate if BRMS1 is a potential CDK substrate, in vitro and in vivo phosphorylation studies were performed. To determine if BRMS1 is phosphorylated in cells, ectopic FLAG-tagged BRMS1 was expressed in HEK-293T cells, which were then metabolically labeled with [32P] orthophosphate. SDS-PAGE of immunoprecipitated FLAG-tagged BRMS1 revealed that this protein is readily phosphorylated in HEK-293T cells (, left, lane 3). BRMS1 phosphorylation was reduced in the presence of a CDK1 and CDK2 inhibitor, RoscotivineCitation52,53 (, left, lane 2), indicating that BRMS1 is a phosphoprotein in cells and that its phosphorylation is dependent on active CDK1/2. Phosphoamino acid analysis of BRMS1 isolated from HEK-293T cells revealed that it is predominantly phosphorylated on serine residue/s (, right panel).
To confirm that BRMS1 is directly phosphorylated by CDKs, we incubated full-length purified recombinant His6-tagged BRMS1 with purified Cyclin A/CDK2 in the presence of [γ32P] ATP in an in vitro phosphorylation reaction. These studies show that BRMS1 is readily phosphorylated by Cyclin A/CDK2 in vitro (, Lane 4). Subsequent phosphoamino acid analysis revealed that BRMS1 is phosphorylated on serine residue/s by Cyclin A/CDK2 in vitro (, right panel). To determine if Cyclin A/CDK2 phosphorylates BRMS1 on serine 237, this site was mutated to alanine (S237A) and subjected to an in vitro kinase assay. While wild-type BRMS1 was readily phosphorylated by Cyclin A/CDK2, under the same conditions BRMS1-S237A was not phosphorylated (, Lanes 4 and 5), indicating that Cyclin A/CDK2 phosphorylates BRMS1 on serine 237.
Finally, mass spectrometry was performed to confirm the phosphorylation site on BRMS1. Mass spectra of peptides derived from BRMS1 phosphorylated by Cyclin A/CDK2 in vitro, following tandem MS/MS confirmed phosphorylation of serine 237 (pS237) on BRMS1 phosphopeptide 234–241 (AAVpSPQKR) (, In Vitro). Mass spectrometry of ectopic BRMS1 immunoprecipitated from BT-549 cells also showed that BRMS1 is phosphorylated on serine 237 in cells (, In Vivo).
Altogether, these studies demonstrate that BRMS1 is directly phosphorylated on serine 237 by Cyclin A/CDK2 in vitro and is phosphorylated on the same site in HEK-293T cells in a CDK-dependent manner (Roscovitine-sensitive). Therefore, BRMS1 is a novel CDK substrate. These findings are consistent with several phosphoproteomic studies showing phosphorylation of BRMS1 serine 237 in various different cell types, as described in the introduction.Citation43-49
Phosphorylation of BRMS1 on serine 237 does not affect cell cycle progression, proliferation or colony formation
Since CDKs play a crucial role in promoting cell division, we investigated if CDK-mediated phosphorylation of BRMS1 may play a role in regulating cell cycle progression. We performed these studies in MDA-MB-231 breast cancer cells, since this is a well characterized metastatic cell line that has been extensively used to study BRMS1 metastasis suppressor function.Citation54-56 MDA-MB-231 breast cancer cell lines stably expressing wild-type BRMS1 (BRMS1-WT), or BRMS1 mutants with serine 237 mutated to alanine (BRMS1-S237A), or aspartate (BRMS1-S237D), were generated following infection with recombinant lentiviruses. The MDA-MB-231 stable cell lines expressed similar levels of BRMS1-WT, BRMS1-S237A and BRMS1-S237D protein and mRNA ( and , left histogram). The BRMS1-S237A mutant with the neutral alanine at position 237 mimics a constitutively non-phosphorylated version of BRMS1. Conversely, the BRMS1-S237D mutant with the negatively charged aspartate at position 237 mimics a constitutively phosphorylated version of BRMS1. To study the potential effects of BRMS1 phosphorylation on cell cycle progression, we initially assessed if expression of BRMS1-WT, BRMS1-S237A or BRMS1-S237D impacts on S phase progression of the cell cycle. Cells were pulsed with 5-bromo-2’-deoxyuridine (BrdU), which incorporates into newly synthesized DNA and the proportion of cells in S-phase were analyzed by fluorescence-activated cytometry (FACS). Expression of BRMS1-WT, BRMS1-S237A and BRMS1-S237D only marginally increased the percentage of cells in S-phase, compared to vector control cells. Therefore, 57.8 % of asynchronous vector control cells were observed in S-phase, compared to 68.7 %, 69.2 % and 65.6 % in S-phase, for cells expressing BRMS1-WT, BRMS1-S237A and BRMS1-S237D, respectively (, left histogram). Although there was an apparent slight increase in the percentage of cells in S phase when all versions of BRMS1 were ectopically expressed, no differences were observed between cells expressing BRMS1-WT, BRMS1-S237A or BRMS1-S237D (, left histogram).
Figure 2. Phosphorylation of BRMS1 on serine 237 does not affect cell cycle progression or proliferation. (A) Western blot representing stable MDA-MB-231 cells expressing (Lane 1) Vector, (Lane 2) BRMS1-WT, (Lane 3) BRMS1-S237A and (Lane 4) BRMS1-S237D. (B, left histogram) Effect of BRMS1-S 237 on S-phase. MDA-MB-231 cells infected with the pLenti-DEST6/V5 (Vector) or expressing BRMS1-WT, BRMS1-S237A or BRMS1-S237D were assessed for the percentage of cells in S phase. Cells were labeled with BrdU, fixed with 70% ethanol, stained with FITC-conjugated anti-BrdU antibody and analyzed by FACS on a Fortessa cell analyzer. Gating was done based on positive staining for BrdU (left panel). The percentage of cells in the S phase is represented in the histogram; Vector (57.8 %), BRMS1-WT (68.7 %), BRMS1-S237A (69.2 %), BRMS1-S237D (65.6 %). One-Way ANOVA with Post-Tukey test were carried out for analysis. Error bars represent ±SEM from 3 biological samples (B, right histogram) Effect of BRMS1-S 237 on M-phase. To measure the percentage of cells in the mitotic phase, cells were subjected to double staining with AlexaFluro 647-conjugated anti-phosphohistone H3 antibody and Propidium Iodide followed by analysis by FACS on a Fortessa cell analyzer. Cells with double the DNA content that positively stained for phosphohistone H3 were gated (left panel). Quantification was performed using One-Way ANOVA with Post-Tukey test. The percentage of cells in mitosis is represented in the histogram; Vector (2.3 %), BRMS1-WT (1.9 %), BRMS1-S237A (2.2 %), BRMS1-S237D (2.1 %). Error bars represent ±SEM from 3 biological samples. (C) Effect of BRMS1-S 237 on cell proliferation. Cells were plated in 96-well plates and their rate of proliferation was quantified by MTS assay over 7 d. Error bars represent ±SEM from 3 biological samples. (D) Effect of BRMS1-S 237 on colony formation. 3000 cells were seeded onto 6 cm plates and left to grow for 7 d. Colonies were fixed and stained with Crystal Violet (left panel) and the number of colonies was determined using ImageJ (Right panel). Error bars represent ±SEM of 3 biological samples.
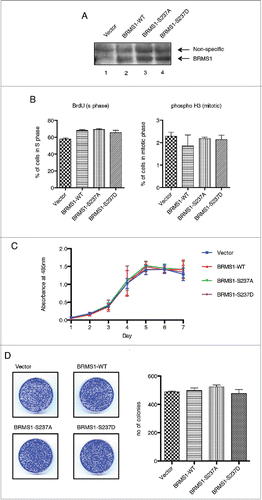
Figure 3. Phosphorylation of BRMS1 on serine 237 regulates cell migration. (A) Scratch-induced migration assay. 90 % confluent MDA-MB-293 cells were scratched with pipette tips. Phase contrast microscope images were taken at 0 and 24 hours post-wound induction from 3 different experiments and the size of the wounds was measured across 5 points, as indicated, and the averages were calculated. The percentage of the initial wound distance covered by the cells after 24h is represented on the histogram (Bottom panel). Error bars represent ±SEM from 3 biological samples. (B) Chemoattractant-induced transwell migration assays. 5 × 104 MDA-MB-231 cells resuspended in 1% FBS supplemented-media were placed in an upper transwell chamber. Cells were then allowed to migrate toward 10% FBS supplemented-media located in the lower chamber for 16 h.Citation67 Following Crystal-Violet staining, non-migratory cells were removed from the inside of the chamber by cotton tips. The stain extracted from the migrating cells was measured at OD560nm using a plate reader. Results were represented as relative fold change compared to control and error bars represent ±SEM from 3 biological samples.
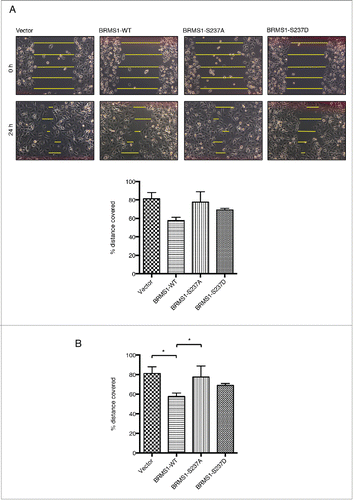
Figure 4. Phosphorylation of BRMS1 on S237 does not affect its association with RBP1 or the mSin3/HDAC complex. (A) Co-immunoprecipitation of RBP1 and BRMS1 mutants. HEK-293T cells were co-transfected with constructs expressing FLAG-tagged RBP1-WT, RBP1-S864A/S1007A or RBP1-S864D/S1007D and Myc-tagged BRMS1-WT, BRMS1-S237A or BRMS1-S237D. Myc-tagged BRMS1 was immunoprecipitated with anti-Myc affinity beads and the co-immunoprecipitated FLAG-tagged RBP1 was detected by Western blotting. Lane 1 represents the control, where the level of non-specific RBP1 binding is assessed on the resin from cells not expressing ectopic BRMS1. Lanes 2–10 represent the levels of the co-immunoprecipiated BRMS1 and RBP1, wild-type or phospho-mutants, as indicated. (B) Co-immunoprecipitation of BRMS1 mutants and mSin3, HDAC1 and SAP30.BT-549 cells were transfected with constructs expressing FLAG-tagged BRMS1-WT (lane 2–3), BRMS1-S237A (lane 4) or BRMS1-S237D (lane 5). 50 μM of the CDK1/2 inhibitor Roscovitine was added to the cells 4 hours prior to lysis, to inhibit the in vivo phosphorylation of BRMS1-WT (Lane 3). BRMS1 was immunoprecipitated from cell lysates with anti- FLAG M2 affinity beads and co-immunoprecipitated mSin3, HDAC1 and SAP30 detected by Western blotting. Lane 1 represents immunoprecipitation from lysates of cells transfected with empty vector.
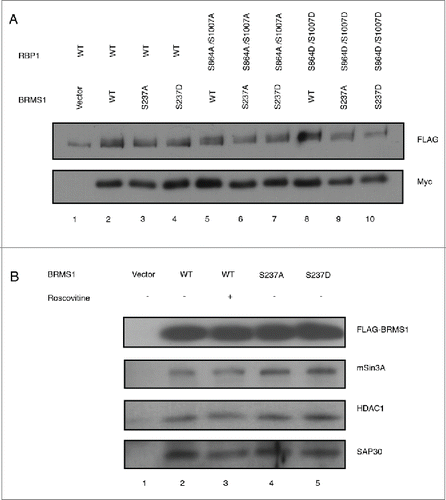
Figure 5. Phosphorylation of BRMS1 serine 237 does not regulate transcriptional repression. (A) Transcriptional regulation of BRMS1-S237. Plasmid expressing Gal4-wild-type-BRMS1, Gal4-BRMS1-S237A or Gal4-BRMS1-S237D were co-transfected into BT-549 cells together with the pGL2-(Gal4)5 TK and pRL-CMV luciferase reporter plasmids. Luminescence of the cell lysates were measured using a plate reader. Firefly luciferase luminescence was normalized against Renilla luciferase luminescence. The graph represents the mean luminescence ±SEM from 3 independent experiments. The western blot (lower panel) demonstrates the levels of BRMS1 expression in these cells. (B) Transcriptional regulation of OPN by BRMS1-S237. qRT-PCR of mRNA from MDA-MB-231 cells expressing BRMS1-WT, BRMS1-S237A, BRMS1-S237D. mRNA levels of, ectopic BRMS1 and OPN were quantified and plotted as relative fold change compared to vector control cells. Error bars represent ±SEM from 3 independent experiments.
We next assessed if expression of the different BRMS1 variants resulted in differences in the proportion of cells in mitosis. Mitotic cells can be identified by evaluating the phosphorylation of histone 3 on serine 10 and serine 28.Citation57,58 Analysis of asynchronous cells for phosphorylated histone 3, revealed that ectopic expression of BRMS1-WT, BRMS1-S237A or S237D did not impact on the proportion of mitotic cells, when compared to vector control cells in this phase of the cell cycle (, right histogram).
If CDK-mediated phosphorylation of BRMS1 serine 237 affects the cell cycle, there may be some impact on cell proliferation. Therefore, we assessed if ectopic expression of the different BRMS1 mutants affected the proliferation of MDA-MB-231 cells. MTS-based cell proliferation assays revealed no differences in proliferation between vector control cells or those expressing BRMS1-WT, BRMS1-S237A or S237D (). Taken together, these results strongly suggest that phosphorylation of BRMS1 on serine 237 does not affect cell cycle progression or cell proliferation.
During metastatic dissemination, cancer cells colonize new secondary sites to develop metastatic tumors. Often, this involves a small number of cells, as the majority of cells do not survive the initial intravasation process, traveling via the lymphatic or circulatory systems and extravasation.Citation59 We conducted colony-forming assays to determine if the phosphorylation of BRMS1 on serine 237 affects the ability of cells to proliferate and generate colonies in isolation in vitro. Similar to the proliferation results (), there were no differences in the number of colonies among MDA-MB-231 cells expressing ectopic BRMS1-WT, BRMS1-S237A or BRMS1-S237D mutants ().
Phosphorylation of BRMS1 on serine 237 affects cell migration
BRMS1 was initially identified as a metastasis suppressor.Citation60 By definition, metastasis suppressors suppress metastasis without affecting the growth of the primary tumor. Metastatic cancer cells disseminate from the primary tumor, colonize distant tissues and organs and grow into secondary metastatic tumors through the processes of migration, intravasation, extravasation and colonization at the new site.Citation61,62 We therefore assessed cell migration, one of the initial steps involved in metastasis. During this process cancer cells undergo an epithelial to mesenchymal transition (EMT), becoming less adherent and more motile.Citation63 We therefore investigated if the phosphorylation state of BRMS1 on S237 affects cell motility by performing wound-healing assays. MDA-MB-231 cells stably expressing either BRMS1-WT or S237A or S237D were grown to a confluent monolayer in 6-well plates. Wounds were introduced by scratching the monolayer using a pipette tip. The ability of the cells to migrate and close the wound was observed 24 hours later. These assays were performed in the presence of 5 mM thymidine to inhibit cell division to ensure that gap closure was due to migration and not cell division.Citation64 Motility was quantified as the percentage of the initial wound distance covered by the cells after 24 h. Consistent with previous studies, expression of wild-type BRMS1 significantly reduced the ability of MDA-MB-231 cells to migrate.Citation65-67 Therefore, while vector control MDA-MB-231 cells covered 81.3 % of the wound area within 24 hours, MDA-MB-231 cells expressing wild-type BRMS1 only covered 57.7% of the wound area (). Cells expressing the BRMS1-S237D mutant covered 69.1% of the wound area, indicating that this mutant also reduced cell motility, albeit not as efficiently as wild-type BRMS1. However, cells expressing the BRMS1-S237A mutant did not inhibit cell motility, with 77.7 % of the wound area covered. These studies suggest that phosphorylation of BRMS1 on serine 237 is important for the ability of this protein to inhibit cell motility.
To further assess the effect of BRMS1 phosphorylation on the migratory ability of cells, we conducted transwell migration assays. Here, serum starved cells were placed onto 8 μM pore transwell chambers and were allowed to migrate toward 10% FBS supplemented media, serving as a chemoattractant. Similar to the wound-healing assays, ectopic expression of BRMS1-WT inhibited the migration of MDA-MB-231, with 54% of the cells migrating through the transwell compared to vector control cells (). Conversely, the migration of the BRMS1-S237A expressing cells was not inhibited with 99% of cells compared to the vector control cells migrating. However, the BRMS1-S237D mutant inhibited transwell migration, with a 78% migration compared to vector cells, although inhibition of migration was not as efficient as that observed with cells expressing BRMS1-WT (). Taken together, these results suggest that the phosphorylation of BRMS1 serine 237 regulates the ability of this protein to control cell migration.
Phosphorylation of BRMS1 serine 237 does not affect its association with RBP1 or the mSin3/HDAC complex
At a molecular level, one of the major functions of BRMS1 is its transcriptional regulation through its interaction with the mSin3/HDAC co-repressor complex.Citation36,39,Citation68-70 Our previous studies have shown that CDK-mediated phosphorylation of pRb and RBP1 disrupts their interaction to dissociate the mSin3/HDAC complex from these proteins during cell cycle progression.Citation21 Since BRMS1 also interacts with RBP1 and the mSin3/HDAC complex,Citation36 we investigated whether CDK-mediated BRMS1 phosphorylation may affect its interaction with the mSin3/HDAC complex.
We first investigated whether the phosphorylation status of BRMS1 affects its association with RBP1. Constructs coding for expression of the phospho-mutants of Myc-tagged BRMS1 and FLAG-tagged RBP1 were co-transfected into HEK-293T cells and the cell lysates were subjected to co-immunoprecipitation. Anti-Myc affinity beads were used to immunoprecipitate Myc-tagged BRMS1 and the level of associated FLAG-tagged RBP1 was detected by Western blotting. Despite detecting small amounts of non-specific binding between FLAG-RBP1 and the empty control resin, Myc-tagged BRMS1 co-immunoprecipitated significantly greater levels of FLAG-RBP1 (, Lanes 1 and 2).Citation36 We then evaluated the association of the various non-phosphorylated (alanine mutants: BRMS1-S237A and RBP1-S864A/S1007A) and constitutively phosphorylated (aspartate mutants: BRMS1-S237D and RBP1-S864D/S1007D) BRMS1 and RBP1 phospho- and non-phospho-mimics. When adjusted for the level of Myc-BRMS1, the levels of the associated FLAG-RBP1 proteins were constant, irrespective of the phosphorylation state and which particular combination of Myc-BRMS1 and FLAG-RBP1 phospho-mutants were examined (, Lanes 2–10). Therefore, phosphorylation of neither RBP1 nor BRMS1 on their respective CDK phosphorylation sites did not affect their association.
We next investigated if the phosphorylation status of BRMS1 affects its association with members of the mSin3/HDAC complex, including mSin3A, HDAC1 and SAP30. BT-549 cells were transfected with constructs expressing FLAG-tagged BRMS1-WT, BRMS1-S237A or BRMS1-S237D. BRMS1 was immunoprecipitated with anti-FLAG M2 affinity beads and the levels of the co-immunoprecipiated mSin3A, HDAC1 and SAP30 were assessed by Western blotting. As expected, mSin3A, HDAC1 and SAP30 specifically co-immunoprecipitated with FLAG-tagged BRMS1-WT, but not with immunoprecipitates from cells transfected with empty vector (, Lanes 1 -2). Addition of the CDK inhibitor Roscotivine, which reduces phosphorylation of BRMS1-WT in vivo (), did not affect the levels of co-immunoprecipitaed mSin3A, HDAC1 and SAP30 (, Lanes 2–3). Immunoprecipitation of the BRMS1-S237A and S237D phospho-mutants also resulted in similar levels of co-immunoprecipiated mSin3A, HDAC1 and SAP30 (, Lanes 2–5). Altogether, these studies indicate that the CDK-mediated phosphorylation of BRMS1 does not affect its association with RBP1, mSin3A, HDAC1 and SAP30.
Phosphorylation of BRMS1 on serine 237 does not affect its transcriptional repression of osteopontin
Our data show that the phosphorylation status of BRMS1 does not affect its association with RBP1, mSin3A, HDAC1 or SAP30 (). However, it is possible that phosphorylation of BRMS1 affects transcription by mechanisms independent of its association with components of the mSin3/HDAC complex, such as through allosteric alterations. To assess this, we performed transcription reporter assays to determine if the phosphorylation of BRMS1 serine 237 altered its transcriptional activity. Constructs were generated where BRMS1-WT, BRMS1-S237A or BRMS1-S237D were fused to the Gal4 DNA-binding domain. Firefly luciferase plasmid containing Gal4 DNA binding domains upstream of the constitutively active thymidine kinase (TK) promoter, which controls downstream expression of Firefly luciferase, was used as reporter.Citation71 For normalization, pRL-CMV plasmid, which encodes for Renilla luciferase gene, was co-transfected into BT-549 cells. These studies showed that wild-type BRMS1 strongly repressed transcription of the luciferase reporter gene, to 8% of that observed in cells transfected with empty vector. Cells expressing BRMS1-S237A and BRMS1-S237D also repressed transcriptional activity, to 20% and 14% of that observed with vector cells, respectively (). Although the level of repression observed with BRMS1-S237A appeared weaker than that induced by BRMS1-WT and BRMS1-S237D, these differences were not statistically significant ().
To further extend these studies, we investigated the transcriptional repression of the pro-metastatic osteopontin (OPN) gene, an established BRMS1 target,Citation39,40 whose increased expression is associated with poor prognosis.Citation72,73 Repression of OPN is mediated in part via the suppression of nuclear-factor κB (NF-κB) activity through the recruitment of HDAC3.Citation39 We investigated the levels of OPN mRNA following ectopic expression of BRMS1-WT, BRMS1-S237A or BRMS1-S237D. These studies showed that BRMS1-WT significantly reduced the levels of OPN mRNA by approximately 94% (). Similarly, expression of BRMS1-S237A and BRMS1-S237D also reduced OPN mRNA by 97% and 94%, respectively (). Therefore, mutation of BRMS1 serine 237 does not affect the transcriptional repression of this protein toward its target gene OPN.
Phosphorylation of BRMS1 on serine 237 does not affect its subcellular localization
Since BRMS1 functions as a transcriptional regulator through binding the mSin3/HDAC complex,Citation36,39,Citation68-70 at least some of its actions are nuclear-mediated. However, recent studies also indicate a cytoplasmic role for BRMS1 in metastasis suppression.Citation54 BRMS1 has 2 NLSs (), however, only the NLS1 sequence (198–205) is important for nuclear localization, while the NLS2 sequence (238–244) is important for metastasis suppression.Citation54 Since BRMS1 serine 237 is located immediately N-terminal to NLS2 and between NLS1 and NLS2 (), phosphorylation of BRMS1 serine 237 may regulate its shuttling between the cytoplasm and nucleus. To investigate if BRMS1 phosphorylation affects its subcellular localization. GFP-tagged BRMS1 constructs were generated, transfected into BT-549 cells for visualization by fluorescence microscopy. BRMS1 levels in the nucleus and cytoplasm were quantified using ImageJ, as previously described.Citation74 There was a relatively equal distribution of BRMS1-WT between the cytoplasm and nucleus (54%:46%). The BRMS1-S237A and BRMS1-S237D mutants had a cytoplasmic:nucleus ratios of 64%:36% and 61%:38%, respectively. These results demonstrated similar distribution of the different BRMS1 variants between the cytoplasm and the nucleus (). Therefore, phosphorylation of BRMS1 on serine 237 does not have a significant effect on its nuclear or cytoplasmic localization.
Figure 6. Phosphorylation of on serine 237 does not affect subcellular localization. Subcellular localization of GFP-tagged BRMS1-WT, BRMS1-S237A or BRMS1-S237D in BT-549 cells. Transfected cells were plated onto cover slips and nuclear staining (Hoechst) and GFP-BRMS1 localization were visualized using am Olympus BX-51 fluorescence microscope. Fluorescence intensities within the cytoplasm and nucleus were expressed as percentages of the total fluorescence in the cell. The total area of each cell was determined based on Differential Interference Contrast (DIC) images while nuclei area was based on the Hoechst stain. Cytoplasmic to nucleus ratios were calculated as previously described [32]. Error bars represent ±SEM of 3 independent experiments.
![Figure 6. Phosphorylation of on serine 237 does not affect subcellular localization. Subcellular localization of GFP-tagged BRMS1-WT, BRMS1-S237A or BRMS1-S237D in BT-549 cells. Transfected cells were plated onto cover slips and nuclear staining (Hoechst) and GFP-BRMS1 localization were visualized using am Olympus BX-51 fluorescence microscope. Fluorescence intensities within the cytoplasm and nucleus were expressed as percentages of the total fluorescence in the cell. The total area of each cell was determined based on Differential Interference Contrast (DIC) images while nuclei area was based on the Hoechst stain. Cytoplasmic to nucleus ratios were calculated as previously described [32]. Error bars represent ±SEM of 3 independent experiments.](/cms/asset/7e07f272-985c-4f42-a2f8-30f8cd9e274f/kccy_a_1121328_f0006_oc.gif)
Discussion
BRMS1 suppresses metastasis in many different types of cancersCitation65,66,Citation75-77 by regulating various steps of the metastasis cascade, such as the adhesion, invasion, cell to cell communication and cell migration.Citation66,78 At a molecular level, BRMS1 mediates its effects through a number of complex mechanisms, including transcriptional regulation of numerous genesCitation41 through its association with the mSin3/HDAC transcriptional repressor complex.Citation36 This includes repression of genes with a known roles in metastasis, such as EGFR, osteopontin and uPA, via the NF-κB pathway.Citation39,42,68 In addition, BRMS1 appears to regulate metastasis through non-transcriptional mechanisms, as a recent study has suggested a cytoplasmic role in BRMS1-mediated metastasis suppression.Citation79 The emerging literature therefore shows that BRMS1 exerts its effects through multiple molecular mechanisms.
While studies have demonstrated that BRMS1 can modulate numerous molecular pathways to suppress metastasis, there has been little information on the upstream regulation of BRMS1 itself. Several studies have reported that BRMS1 expression can be regulated through methylation of its promoter.Citation80,81 Reduced BRMS1 expression is associated with advanced pathology and poor survival of non-small cell lung cancer patientsCitation80,81 and reduced disease-free survival rate in breast cancer sufferers.Citation82,83 In addition to promoter methylation, the activity of BRMS1 is negatively regulated by E3 ligase activity.Citation84
Our studies demonstrate that in addition to epigenetic control, BRMS1 activity is regulated at a post-translational level through phosphorylation. Our in vitro and in vivo phosphorylation studies indicate that BRMS1 is phosphorylated on serine 237 by CDKs. This is in agreement with several emerging phosphoproteomics studies demonstrating phosphorylation of BRMS1 on serine 237 in different cell lines, such as HeLa cervical cancer cells, liver cells, embryonic stem cells, acute myeloid leukemia cells and fibroblasts.Citation43-49 Therefore, phosphorylation of BRMS1 on serine 237 appears to be widespread, however, the responsible kinase/s and functional consequences were not investigated. CDKs are best described for their major role in promoting cell cycle progression through phosphorylation of important cell cycle regulatory protein substrates.Citation3,21,85,86 Our studies strongly suggest that phosphorylation of BRMS1 does not affect cell cycle progression or cell proliferation. Therefore, MDA-MB-231 cells stably expressing wild-type or constitutively non-phosphorylated (BRMS1-S237A) or constitutively phosphorylated (BRMS1-S237D) BRMS1 mimics displayed similar cell cycle profiles, rates of cell proliferation and colony formation capabilities (). Consistent with previous studies,Citation65-67 ectopic expression of BRMS1-WT suppressed the motility of MDA-MB-231 in transwell migration and wound healing assays (). Our studies demonstrated that the BRMS1 constitutive phospho-mimic, BRMS1-S237D, also inhibited migration, albeit not as efficiently as wild-type BRMS1. However, it should be appreciated that replacement of serine 237 with aspartate is a mimic and may not be as effective as a negatively charged phosphoserine. Conversely, the constitutively non-phosphorylated mimic, BRMS1-S237A, did not inhibit the motility and migration of cells (). These studies demonstrate that phosphorylation of BRMS1 on serine 237 is necessary for inhibiting the migration of MDA-MB-231 cells.
The emerging literature indicates that CDKs can regulate numerous processes distinct from cell cycle control. For example, neuronal CDK5 plays an important role in synaptic vesicle recycling through phosphorylation of Dynamin I,Citation87 while the phosphorylation of LRP6 co-receptor by Cyclin Y/CDK14 complex promotes Wnt signaling.Citation88 CDKs have also been implicated in epigenetic regulation. The phosphorylation of Enhancer of Zeste homolog 2 (EZH2) on Threonine 350 by CDK1/2 enhances its methyltransferase activity, which results in the suppression of its target genes.Citation89 On the other hand, phosphorylation of EZH2 on Threonine 487 disrupts its binding to the catalytic subunit of Polycomb repressive complex 2 (PRC2) and targets it for ubiquitin-mediated proteolytic degradation.Citation90,91 CDKs can also regulate metabolism. For example, CDK5 and CDK8 regulate glycogenesis and lipogenesis, respectively.Citation92,93
It is now well established that overactive and deregulated CDK activities contribute to tumorigenesis, due to the promotion of uncontrolled proliferation of cancer cells.Citation94-97 Our studies now indicate that CDKs may directly regulate cell migration through phosphorylation of a metastasis suppressor, adding to the repertoire of functions regulated by CDKs. In agreement with our findings, recent studies suggest that CDKs can control the migration and metastatic potential of cancer cells. For example, Cyclin A regulates the RhoA signaling pathway,Citation98 which plays a major role in cellular movement. Inhibition of CDK4/6 activity with PD-0332991 or their knockdown with shRNA, inhibited the growth of pancreatic cancer cells, but enhanced their invasion.Citation99 This was associated with a reduced expression of cell cycle regulatory genes, but increased expression of genes involved in extracellular matrix remodeling, invasion and metastasis. CDK4/6 inhibition also induced EMT, which represents one of the initial stages of metastasis, where cells transition from an epithelial to mesenchymal phenotype, becoming less proliferative and less adherent, but more motile and invasive.Citation100,101 Consistent with these studies, downregulation of Cyclin D1 in breast cancer cells decreased their proliferation, but increased their migrationCitation102,103 and this was associated with EMT.Citation104 During Snail/Slug and ZEB1/SIP1 induced EMT, SIP1 induces a decrease in Cyclin D1 gene expression and reduced cell cycle progression.Citation104 Therefore, the emerging paradigm suggests that cancer cells at the primary tumor site, are predominantly epithelial and those with deregulated Cyclin/CDK activity undergo uncontrolled proliferation. Upon EMT, Cyclin/CDK activity is downregulated and the proliferation of cancer cells is reduced, but their motility and invasiveness is increased. Our results suggest that CDK-mediated phosphorylation of BRMS1 is one pathway which contributes to a less motile phenotype of epithelial cancer cells in the primary tumor. Similar findings have been reported for the CDK substrate, Smad3.Citation105 Smad3 is a transcriptional regulator, which mediates the anti-proliferative effects of TGF-β. While CDK-mediated phosphorylation of Smad3 reduces the cell cycle inhibitory effect of this protein to promote cell proliferation, it is also important for reducing metastasis of breast cancer cells.Citation105 Therefore, mutation of the CDK phosphorylation sites in the linker region of Smad3, significantly increased lung cancer metastases in CA1a and 4T1 breast cancer cells. Therefore, high CDK activity can concomitantly contribute to increased proliferation and reduced migration of cancer cells.
How CDK-mediated phosphorylation regulates BRMS1 effects on cell migration is not clear. Our biochemical studies indicate that BRMS1 phosphorylation does not affect its association with the components of the mSin3/HDAC complex or RBP1 (). Consistent with these results, phosphorylation did not affect transcriptional repression mediated by BRMS1, as demonstrated by transcription reporter assays () or evaluation of transcriptional repression of its target gene, osteopontin (). Interestingly, the BRMS1 serine 237 phosphorylation site is located immediately N-terminal to the predicted NLS2 (amino acids 238–244) (). BRMS1 also contains a predicted NLS1 (amino acids 198–205) (), but only NLS1 is important for nuclear localization of BRMS1.Citation54,106 Consistent with these findings, we showed that mutation of serine BRMS1 237 did not impact its cytoplasmic/nuclear distribution (). However, NLS2 plays a critical role in metastasis suppression, and mediates BRMS1 association with mSin3 complexes in the cytoplasm.Citation54 In addition, this NLS2 region is important for down regulation of the pro-metastatic microRNA miR-10b. Therefore, CDK-mediated phosphorylation of BRMS1 may be regulating the cytoplasmic functions of this protein, which awaits further investigation.
In conclusion, our study has now identified BRMS1 as a novel CDK substrate, whose phosphorylation regulates the migration of breast cancer cells in vitro. This work deepens our understanding on the role of CDKs during normal cell cycle progression and how deregulated CDK activity can control different aspects of carcinogenesis.
Experimental procedures
Plasmids, cell lines and antibodies
Human BRMS1 (BC009834) cDNA was purchased from Open Biosystems, USA (clone ID# 3959486). BRMS1 cDNA was cloned into the pCMV Tag2A mammalian expression vector to produce N-terminal FLAG-tag recombinant protein. Site-directed mutagenesis was performed to generate phospho-mutants, BRMS1-serine-237A-alanine (BRMS1-S237A) and BRMS1-serine 237-aspartate (BRMS1-S237D). BRMS1 wild-type and phosphor-mutants were subcloned into pLenti6/V5-DEST vectors. Lentivirus packaging plasmids, VSVg, pCMV-delta8.9 were donated by Dr. Mark Chong, St Vincent's Institute, Melbourne, Australia. BRMS1-WT, BRMS1-S237A and BRMS1-S237D cDNA were subcloned into pET-15b for bacterial expression of N-terminal His-tagged proteins, and pEGFP-C2 for N-terminal eGFP-tagged recombinant proteins.
Human embryonic kidney 293 cells expressing large-T antigen (HEK-293T) were maintained in Dulbecco's Modified Eagle's Medium (DMEM) (Lonza) supplemented with 10% Fetal Bovine Serum (Thermofisher). The human breast cancer cell lines BT-549, MDA-MB-231 and stable MDA-MB-231 cell lines expressing wild-type BRMS1, BRMS1-S237A and BRMS1- S237D, following infection with pLenti-DEST6/V5 recombinant lentiviruses, were maintained in Roswell Park Memorial Institute-1640 (RPMI-1640) (Lonza) medium, supplemented with 10% FBS. All cells were cultured at 37°C with 5% CO2.
Mouse anti-FLAG monoclonal antibody (M2, Sigma, F1804) and anti-Myc epitope (9E10, Santa Cruz, sc-40), rabbit anti-human SAP30 (Upstate, 06–875), anti-human HDAC1 (H-51, Santa Cruz, sc- 7872) and anti- human mSin3A (AK-11) (Santa Cruz, sc-767) polyclonal antibodies were used according to manufacturer's instructions. Rabbit anti-BRMS1 polyclonal antibodies were generated in house and affinity purified with protein-A-sepharose.
Expression and purification of recombinant proteins
Recombinant His6-BRMS1-WT, BRMS1-S237A and BRMS1-S237D were expressed in E. coli strain Rosetta BL21 (DE3) pLysS (Novagen). Cultures were grown in LB medium containing 100 μg/ml ampicillin and 50 μg/ml chloramphenicol to OD600 = 0.8 and protein expression was induced by 1 mM isopropyl-γ-D-thiogalactoside (IPTG). The cell lysate containing denatured His6-BRMS1 was applied onto a column containing Ni2+-NTA resin and washed with gradual reduction in Urea concentrations. His6-BRMS1 was eluted with imidazole elution buffer and dialyzed against dialysis buffer (50 mM HEPES pH 7.5, 1 mM dithiothreitol (DTT), 0.01% Tween-20, 5% glycerol, 0.5 mM phenylmethylsulfonyl fluoride (PMSF).
In vivo and in vitro phosphorylation
Asynchronous HEK293T cells were transfected with pCMV-Tag2A-BRMS1, labeled with [32P] orthophosphate (MP Biomedicals), and BRMS1 phosphorylation analyzed as described previously.Citation21 Recombinant His6-tagged BRMS1-WT, BRMS1-S237A and BRMS1-S237D, purified from E.coli and recombinant Cyclin A/CDK2 were used for in vitro phosphorylation. His6-BRMS1 was incubated with recombinant Cyclin A/CDK2 in kinase reaction buffer (17 mM HEPES pH 7.5, 10 mM MgCl2, 0.3 mM DTT, 10 mM NaF, 10 mM β–glycerophosphate, 1 mM vanadate, 100 μM ATP, 5 μCi [γ- 32P] ATP) at 37°C for 1h. The reaction was stopped with SDS-Laemmli buffer and samples resolved by SDS-PAGE, prior to autoradiography. Phosphoamino acid analysis was performed as described previously.Citation21
Mass spectrometry analysis
in vitro and in vivo phosphorylated His6 and FLAG-tagged BRMS1 proteins were resolved by SDS-PAGE, excised from the gel and digested with trypsin (Promega). Peptide extraction, trypsin digestion and mass spectrometry analysis was performed as described previously.Citation21
Lentiviral infection
HEK-239T cells were used to produce lentiviral stocks as previously described.Citation107,108 Following infection of MDA-MB-231 cells with these lentiviral stocks, stably expressing cells were selected using 5 μg/ml Blasticidin (InvivoGen, ant-bl-1).
Cell cycle studies
MDA-MB-231 cells stably expressing BRMS1 wild-type or phosphiomutants were plated 24 h prior to their collection. Trypsinised cells were fixed with 70% ethanol overnight at 4°C, washed and resuspended in 100 μl of FACS buffer containing 50 μg RNAse A and 8 μg propidium iodide (PI) (Sigma). Samples were incubated at 37°C in the dark for 30 min, washed and stained with AlexaFluor 647-conjugated anti-phosphohistone H3 antibody (BD Pharmingen) at RT in the dark for 20 min. For BrdU labeling, cells were incubated with BrdU (1 μg/ml) (Sigma) 2h prior to collection. Fixed cells were stained with FITC-conjugated anti- BrdU antibody (BD Biosciences), as previously describedCitation21 and were analyzed on FACS Fortessa.
Cell proliferation assay
500 MDA-MB-231 cells stably expressing BRMS1 were plated in 96-well plates. Every day, for 7 days, 20 μl of MTS (Cell Titre 96 proliferation assay, Promega) were added to each well. Plates were left at 37°C, with 5% CO2, for 4 hours before reading absorbance at 485 nm using a plate reader (Polarstar).
Wound healing assay
5 × 105 MDA-MB-231 cells stably expressing BRMS1 were plated in 6-well plates 24 h prior to assay. Sterile P200 tips were used to scratch and create gaps in the confluent cell monolayer. To preclude effects of proliferation, 5 mM Thymidine was added to arrest cell cycle progression. Images of the wounded monolayers were taken at 0 and 24 h and the size of the wounds was measured using ImageJ.
Transwell migration assay
MDA-MB-231 cells stably expressing BRMS1 were serum-starved for 24 h. Cells were trypsinized and resuspended in 1% FBS-supplemented RMP1-1640. 5 × 104 cells were placed in the upper chamber, 6.5 mm diameter with 8 μm pores and incubated at 37°C with 5% CO2 for 2 h, to allow for settlement. The Chemoattractant (10% FBS-supplemented RMP1–1640) was placed in the lower chamber and the cells were allowed to migrate for 16 h, to minimize effects of proliferation (doubling time ∼24 h). Analysis of migrated cells was performed as per the manufacturer's protocol (Colorimetric QCM Chemotaxis Cell Migration Assay, 24-well (8 μm), Cat# ECM508, Merck Millipore).
Colony-formation assay
– 3 × 103 MDA-MB-231 cells stably expressing BRMS1 were plated onto 6 cm plates with 4 ml RPMI-1640 and left to grow for 7 d. Following this, cells were washed with PBS and stained with Crystal Violet [0.25% Crystal Violet, 4% Paraformaldehyde (PFA)]. The plates were extensively washed with PBS and left to dry overnight. The number of colonies was determined using ImageJ.
Co-immunoprecipitation of BRMS1, RBP1, mSin3A, HDAC1 and SAP30
Cell lysates from BT-549 transiently transfected with BRMS1 +/- RBP1 were mixed with anti-FLAG M2 affinity beads (Sigma) at 4°C, overnight. The beads were washed extensively and samples resolved by SDS-PAGE. Western blot analysis carried out using antibodies against human mSin3A, human HDAC1, human SAP30, Myc or FLAG epitopes to detect BRMS1 or RBP1.
Subcellular localization
BT-549 cells were plated onto coverslips 24 h prior to transfection with pEGFP-C2 expressing BRMS1-WT, BRMS1-S237A or BRMS1-S237D using FugeneHD (Roche). 24 h post-transfection, cells were washed with cold PBS, fixed with 2% PFA for 5 min followed by washing with PBS and nuclei staining with Hoerscht Stain for 15 min. Cells were then washed twice with PBS and mounted onto slides. Images were acquired using Olympus BX-51 fluorescence microscope and fluorescence was measured using ImageJ.
Dual-luciferase transcription reporter assays
Transcriptional reporter assays were performed using the Dual-Luciferase Reporter Assay kit (Promega) according to the manufacturer's instructions. Briefly, pGL2-(Gal4)5 TK Firefly luciferase and pRL-CMV Renilla luciferase plasmid were transiently co-transfected with either pCDNA3.1-Gal4, pCDNA3.1-Gal4-BRMS1, pCDNA3.1-Gal4-BRMS1-S237A or pCDNA3.1-Gal4-BRMS1-S237D plasmids into BT-549 or HEK-293T and incubated for 48 h. Cell lysates were treated as per manufacturer's recommendations. Luminescence from both Firefly and Renilla luciferase were measured using a microplate reader (Polarstar)
Quantitative real-time PCR
Total RNA from MDA-MB-231 cells stably expressing BRMS1-WT, BRMS1-S237A or BRMS1-S237D was isolated using NucleoSpin® RNA II kit (Macherey-Nagel). RNA was reverse transcribed to cDNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystem). Each sample was then subjected to Real-time PCR analysis using the Taqman® Gene Expression Assay for OPN (Hs00167093_m1) and β-actin (Hs99999903_m1) was used for housekeeping reference gene. Analysis was performed using a LightCycler 480 (Roche).
Disclosure of potential conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Author contributions
SNAR, RS, EM and ART designed, performed and analyzed experiments in and contributed to writing the manuscript. SMAI and JSO performed the mass spectrometry experiments in . OB, DRW and BS conceived the study, coordinated and wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Acknowledgments
We thank Dr Carl Walkey for his extensive help with production of the lentivirus stocks, Lina Mariana, Dr Fenil Shah and Dr Andrew Deans for advice on various experimental techniques and input into cloning strategies.
Funding
This research was supported by grants from the National Health and Medical Research Council (1011320) to B.S and the National Foundation for Cancer Research (DRW). We acknowledge the support of the Victorian State Government Operational Infrastructure Support Program to St. Vincent's Institute.
References
- Stoltz RA, Conners MS, Gerritsen ME, Abraham NG, Laniado-Schwartzman M. Direct stimulation of limbal microvessel endothelial cell proliferation and capillary formation in vitro by a corneal-derived eicosanoid. Am J Pathol 1996; 148:129-39; PMID:8546200
- Scher CD, Stiles CD, Antoniades HN, Pledger WJ. Regulation of the mammalian fibroblast cell cycle by a platelet-derived growth factor. Prog Clin Biol Res 1979; 31:611-20; PMID:538019
- Suryadinata R, Sadowski M, Sarcevic B. Control of cell cycle progression by phosphorylation of cyclin-dependent kinase (CDK) substrates. Biosci Rep 2010; 30:243-55; PMID:20337599; http://dx.doi.org/10.1042/BSR20090171
- Tam SW, Theodoras AM, Shay JW, Draetta GF, Pagano M. Differential expression and regulation of Cyclin D1 protein in normal and tumor human cells: association with Cdk4 is required for Cyclin D1 function in G1 progression. Oncogene 1994; 9:2663-74; PMID:8058330
- Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol 1994; 14:2077-86; PMID:8114739; http://dx.doi.org/10.1128/MCB.14.3.2077
- Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR, Roberts JM. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science 1992; 257:1689-94; PMID:1388288; http://dx.doi.org/10.1126/science.1388288
- Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J 1992; 11:961-71; PMID:1312467
- Draetta G, Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell 1988; 54:17-26; PMID:3289755; http://dx.doi.org/10.1016/0092-8674(88)90175-4
- Jessus C, Beach D. Oscillation of MPF is accompanied by periodic association between cdc25 and cdc2-cyclin B. Cell 1992; 68:323-32; PMID:1310257; http://dx.doi.org/10.1016/0092-8674(92)90473-P
- Mihara K, Cao XR, Yen A, Chandler S, Driscoll B, Murphree AL, T'Ang A, Fung YK. Cell cycle-dependent regulation of phosphorylation of the human retinoblastoma gene product. Science 1989; 246:1300-3; PMID:2588006; http://dx.doi.org/10.1126/science.2588006
- Goodrich DW, Wang NP, Qian YW, Lee EY, Lee WH. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell 1991; 67:293-302; PMID:1655277; http://dx.doi.org/10.1016/0092-8674(91)90181-W
- Hiebert SW, Chellappan SP, Horowitz JM, Nevins JR. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev 1992; 6:177-85; PMID:1531329; http://dx.doi.org/10.1101/gad.6.2.177
- Johnson DG, Schwarz JK, Cress WD, Nevins JR. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature 1993; 365:349-52; PMID:8377827; http://dx.doi.org/10.1038/365349a0
- Lee C, Chang JH, Lee HS, Cho Y. Structural basis for the recognition of the E2F transactivation domain by the retinoblastoma tumor suppressor. Genes Dev 2002; 16:3199-212; PMID:12502741; http://dx.doi.org/10.1101/gad.1046102
- Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature 1998; 391:597-601; PMID:9468139; http://dx.doi.org/10.1038/35404
- Luo RX, Postigo AA, Dean DC. Rb interacts with histone deacetylase to repress transcription. Cell 1998; 92:463-73; PMID:9491888; http://dx.doi.org/10.1016/S0092-8674(00)80940-X
- Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain JP, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature 1998; 391:601-5; PMID:9468140; http://dx.doi.org/10.1038/35410
- Hassig CA, Schreiber SL. Nuclear histone acetylases and deacetylases and transcriptional regulation: HATs off to HDACs. Curr Opin Chem Biol 1997; 1:300-8; PMID:9667866; http://dx.doi.org/10.1016/S1367-5931(97)80066-X
- Lai A, Kennedy BK, Barbie DA, Bertos NR, Yang XJ, Theberge MC, Tsai SC, Seto E, Zhang Y, Kuzmichev A, et al. RBP1 recruits the mSIN3-histone deacetylase complex to the pocket of retinoblastoma tumor suppressor family proteins found in limited discrete regions of the nucleus at growth arrest. Mol Cell Biol 2001; 21:2918-32; PMID:11283269; http://dx.doi.org/10.1128/MCB.21.8.2918-2932.2001
- Lai A, Lee JM, Yang WM, DeCaprio JA, Kaelin WG Jr., Seto E, Branton PE. RBP1 recruits both histone deacetylase-dependent and -independent repression activities to retinoblastoma family proteins. Mol Cell Biol 1999; 19:6632-41; PMID:10490602; http://dx.doi.org/10.1128/MCB.19.10.6632
- Suryadinata R, Sadowski M, Steel R, Sarcevic B. Cyclin-dependent kinase-mediated phosphorylation of RBP1 and pRb promotes their dissociation to mediate release of the SAP30.mSin3.HDAC transcriptional repressor complex. J Biol Chem 2011; 286:5108-18; PMID:21148318; http://dx.doi.org/10.1074/jbc.M110.198473
- Dahiya A, Gavin MR, Luo RX, Dean DC. Role of the LXCXE binding site in Rb function. Mol Cell Biol 2000; 20:6799-805; PMID:10958676; http://dx.doi.org/10.1128/MCB.20.18.6799-6805.2000
- Veitia RA, Ottolenghi C, Bissery MC, Fellous A. A novel human gene, encoding a potential membrane protein conserved from yeast to man, is strongly expressed in testis and cancer cell lines. Cytogenet Cell Genet 1999; 85:217-20; PMID:10449901; http://dx.doi.org/10.1159/000015296
- Ng HH, Bird A. Histone deacetylases: silencers for hire. Trends Biochem Sci 2000; 25:121-6; PMID:10694882; http://dx.doi.org/10.1016/S0968-0004(00)01551-6
- Zhang Y, Iratni R, Erdjument-Bromage H, Tempst P, Reinberg D. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell 1997; 89:357-64; PMID:9150135; http://dx.doi.org/10.1016/S0092-8674(00)80216-0
- Fleischer TC, Yun UJ, Ayer DE. Identification and characterization of three new components of the mSin3A corepressor complex. Mol Cell Biol 2003; 23:3456-67; PMID:12724404; http://dx.doi.org/10.1128/MCB.23.10.3456-3467.2003
- Nicolas E, Morales V, Magnaghi-Jaulin L, Harel-Bellan A, Richard-Foy H, Trouche D. RbAp48 belongs to the histone deacetylase complex that associates with the retinoblastoma protein. J Biol Chem 2000; 275:9797-804; PMID:10734134; http://dx.doi.org/10.1074/jbc.275.13.9797
- Qian YW, Lee EY. Dual retinoblastoma-binding proteins with properties related to a negative regulator of ras in yeast. J Biol Chem 1995; 270:25507-13; PMID:7503932; http://dx.doi.org/10.1074/jbc.270.43.25507
- Chen PL, Scully P, Shew JY, Wang JY, Lee WH. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell 1989; 58:1193-8; PMID:2673546; http://dx.doi.org/10.1016/0092-8674(89)90517-5
- Buchkovich K, Duffy LA, Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell 1989; 58:1097-105; PMID:2673543; http://dx.doi.org/10.1016/0092-8674(89)90508-4
- Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol 1998; 18:753-61; PMID:9447971; http://dx.doi.org/10.1128/MCB.18.2.753
- Zarkowska T, Mittnacht S. Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem 1997; 272:12738-46; PMID:9139732; http://dx.doi.org/10.1074/jbc.272.19.12738
- Burke JR, Deshong AJ, Pelton JG, Rubin SM. Phosphorylation-induced conformational changes in the retinoblastoma protein inhibit E2F transactivation domain binding. J Biol Chem 2010; 285:16286-93; PMID:20223825; http://dx.doi.org/10.1074/jbc.M110.108167
- Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell 1999; 98:859-69; PMID:10499802; http://dx.doi.org/10.1016/S0092-8674(00)81519-6
- Takaki T, Fukasawa K, Suzuki-Takahashi I, Hirai H. Cdk-mediated phosphorylation of pRB regulates HDAC binding in vitro. Biochem Biophys Res Commun 2004; 316:252-5; PMID:15003538; http://dx.doi.org/10.1016/j.bbrc.2004.02.044
- Meehan WJ, Samant RS, Hopper JE, Carrozza MJ, Shevde LA, Workman JL, Eckert KA, Verderame MF, Welch DR. Breast cancer metastasis suppressor 1 (BRMS1) forms complexes with retinoblastoma-binding protein 1 (RBP1) and the mSin3 histone deacetylase complex and represses transcription. J Biol Chem 2004; 279:1562-9; PMID:14581478; http://dx.doi.org/10.1074/jbc.M307969200
- Seraj MJ, Samant RS, Verderame MF, Welch DR. Functional evidence for a novel human breast carcinoma metastasis suppressor, BRMS1, encoded at chromosome 11q13. Cancer Res 2000; 60:2764-9; PMID:10850410
- Roesley SNA, Suryadinata R, Sarcevic B. Metastasis suppressors and their roles in cancer. Cancer Forum 2014:90
- Samant RS, Clark DW, Fillmore RA, Cicek M, Metge BJ, Chandramouli KH, Chambers AF, Casey G, Welch DR, Shevde LA. Breast cancer metastasis suppressor 1 (BRMS1) inhibits osteopontin transcription by abrogating NF-kappaB activation. Molecular cancer 2007; 6:6; PMID:17227585; http://dx.doi.org/10.1186/1476-4598-6-6
- Cicek M, Fukuyama R, Welch DR, Sizemore N, Casey G. Breast cancer metastasis suppressor 1 inhibits gene expression by targeting nuclear factor-kappaB activity. Cancer Res 2005; 65:3586-95; PMID:15867352; http://dx.doi.org/10.1158/0008-5472.CAN-04-3139
- Champine PJ, Michaelson J, Weimer BC, Welch DR, DeWald DB. Microarray analysis reveals potential mechanisms of BRMS1-mediated metastasis suppression. Clin Exp Metast 2007; 24:551-65; PMID:17896182; http://dx.doi.org/10.1007/s10585-007-9092-8
- Vaidya KS, Harihar S, Phadke PA, Stafford LJ, Hurst DR, Hicks DG, Casey G, DeWald DB, Welch DR. Breast cancer metastasis suppressor-1 differentially modulates growth factor signaling. J Biol Chem 2008; 283:28354-60; PMID:18664570; http://dx.doi.org/10.1074/jbc.M710068200
- Weber C, Schreiber TB, Daub H. Dual phosphoproteomics and chemical proteomics analysis of erlotinib and gefitinib interference in acute myeloid leukemia cells. J Proteomics 2012; 75:1343-56; PMID:22115753; http://dx.doi.org/10.1016/j.jprot.2011.11.004
- Phanstiel DH, Brumbaugh J, Wenger CD, Tian S, Probasco MD, Bailey DJ, Swaney DL, Tervo MA, Bolin JM, Ruotti V, et al. Proteomic and phosphoproteomic comparison of human ES and iPS cells. Nat Methods 2011; 8:821-7; PMID:21983960; http://dx.doi.org/10.1038/nmeth.1699
- Rigbolt KT, Prokhorova TA, Akimov V, Henningsen J, Johansen PT, Kratchmarova I, Kassem M, Mann M, Olsen JV, Blagoev B. System-wide temporal characterization of the proteome and phosphoproteome of human embryonic stem cell differentiation. Sci Signal 2011; 4:rs3; PMID:21406692; http://dx.doi.org/10.1126/scisignal.2001570
- Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal 2010; 3:ra3; PMID:20068231; http://dx.doi.org/10.1126/scisignal.2000475
- Sharma K, D'Souza RC, Tyanova S, Schaab C, Wisniewski JR, Cox J, Mann M. Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep 2014; 8:1583-94; PMID:25159151; http://dx.doi.org/10.1016/j.celrep.2014.07.036
- Shiromizu T, Adachi J, Watanabe S, Murakami T, Kuga T, Muraoka S, Tomonaga T. Identification of missing proteins in the neXtProt database and unregistered phosphopeptides in the PhosphoSitePlus database as part of the Chromosome-centric Human Proteome Project. J Proteome Res 2013; 12:2414-21; PMID:23312004; http://dx.doi.org/10.1021/pr300825v
- Bian Y, Song C, Cheng K, Dong M, Wang F, Huang J, Sun D, Wang L, Ye M, Zou H. An enzyme assisted RP-RPLC approach for in-depth analysis of human liver phosphoproteome. J Proteomics 2014; 96:253-62; PMID:24275569; http://dx.doi.org/10.1016/j.jprot.2013.11.014
- Songyang Z, Blechner S, Hoagland N, Hoekstra MF, Piwnica-Worms H, Cantley LC. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr Biol 1994; 4:973-82; PMID:7874496; http://dx.doi.org/10.1016/S0960-9822(00)00221-9
- Songyang Z, Lu KP, Kwon YT, Tsai LH, Filhol O, Cochet C, Brickey DA, Soderling TR, Bartleson C, Graves DJ, et al. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Cell Biol 1996; 16:6486-93; PMID:8887677; http://dx.doi.org/10.1128/MCB.16.11.6486
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem 1997; 243:527-36; PMID:9030781; http://dx.doi.org/10.1111/j.1432-1033.1997.t01-2-00527.x
- Vesely J, Havlicek L, Strnad M, Blow JJ, Donella-Deana A, Pinna L, Letham DS, Kato J, Detivaud L, Leclerc S, et al. Inhibition of cyclin-dependent kinases by purine analogues. Eur J Biochem 1994; 224:771-86; PMID:7925396; http://dx.doi.org/10.1111/j.1432-1033.1994.00771.x
- Hurst DR, Xie Y, Thomas JW, Liu J, Edmonds MD, Stewart MD, Welch DR. The C-terminal putative nuclear localization sequence of breast cancer metastasis suppressor 1, BRMS1, is necessary for metastasis suppression. PLoS One 2013; 8:e55966; PMID:23390556; http://dx.doi.org/10.1371/journal.pone.0055966
- Hurst DR, Edmonds MD, Scott GK, Benz CC, Vaidya KS, Welch DR. Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Res 2009; 69:1279-83; PMID:19190326; http://dx.doi.org/10.1158/0008-5472.CAN-08-3559
- Zhang Y, Ye L, Tan Y, Sun P, Ji K, Jiang WG. Expression of breast cancer metastasis suppressor-1, BRMS-1, in human breast cancer and the biological impact of BRMS-1 on the migration of breast cancer cells. Anticancer Res 2014; 34:1417-26; PMID:24596389
- Gurley LR, D'Anna JA, Barham SS, Deaven LL, Tobey RA. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem 1978; 84:1-15; PMID:206429; http://dx.doi.org/10.1111/j.1432-1033.1978.tb12135.x
- Goto H, Tomono Y, Ajiro K, Kosako H, Fujita M, Sakurai M, Okawa K, Iwamatsu A, Okigaki T, Takahashi T, et al. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem 1999; 274:25543-9; PMID:10464286; http://dx.doi.org/10.1074/jbc.274.36.25543
- Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 1998; 153:865-73; PMID:9736035; http://dx.doi.org/10.1016/S0002-9440(10)65628-3
- Phillips KK, Welch DR, Miele ME, Lee JH, Wei LL, Weissman BE. Suppression of MDA-MB-435 breast carcinoma cell metastasis following the introduction of human chromosome 11. Cancer Res 1996; 56:1222-7; PMID:8640802
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 2006; 12:895-904; PMID:16892035; http://dx.doi.org/10.1038/nm1469
- Leber MF, Efferth T. Molecular principles of cancer invasion and metastasis (review). Int J Oncol 2009; 34:881-95; PMID:19287945
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2:442-54; PMID:12189386; http://dx.doi.org/10.1038/nrc822
- Bootsma D, Budke L, Vos O. Studies on synchronous division of tissue culture cells initiated by excess thymidine. Exp Cell Res 1964; 33:301-9; PMID:14109144; http://dx.doi.org/10.1016/S0014-4827(64)81035-1
- Zhang S, Lin QD, Di W. Suppression of human ovarian carcinoma metastasis by the metastasis-suppressor gene, BRMS1. Int J Gynecol Cancer 2006; 16:522-31; PMID:16681721; http://dx.doi.org/10.1111/j.1525-1438.2006.00547.x
- Mei P, Bai J, Shi M, Liu Q, Li Z, Fan Y, Zheng J. BRMS1 suppresses glioma progression by regulating invasion, migration and adhesion of glioma cells. PLoS One 2014; 9:e98544; PMID:24879377; http://dx.doi.org/10.1371/journal.pone.0098544
- Samant RS, Seraj MJ, Saunders MM, Sakamaki TS, Shevde LA, Harms JF, Leonard TO, Goldberg SF, Budgeon L, Meehan WJ, et al. Analysis of mechanisms underlying BRMS1 suppression of metastasis. Clin Exp Metast 2000; 18:683-93; PMID:11827072; http://dx.doi.org/10.1023/A:1013124725690
- Cicek M, Fukuyama R, Cicek MS, Sizemore S, Welch DR, Sizemore N, Casey G. BRMS1 contributes to the negative regulation of uPA gene expression through recruitment of HDAC1 to the NF-kappaB binding site of the uPA promoter. Clin Exp Metast 2009; 26:229-37; PMID:19165610; http://dx.doi.org/10.1007/s10585-009-9235-1
- Liu Y, Smith PW, Jones DR. Breast cancer metastasis suppressor 1 functions as a corepressor by enhancing histone deacetylase 1-mediated deacetylation of RelA/p65 and promoting apoptosis. Mol Cell Biol 2006; 26:8683-96; PMID:17000776; http://dx.doi.org/10.1128/MCB.00940-06
- Edmonds MD, Hurst DR, Vaidya KS, Stafford LJ, Chen D, Welch DR. Breast cancer metastasis suppressor 1 coordinately regulates metastasis-associated microRNA expression. Int J Cancer 2009; 125:1778-85; PMID:19585508; http://dx.doi.org/10.1002/ijc.24616
- Allard S, Kopish K. Luciferase reporter assays: Powerful, adaptable tools for cell biology research. Cell Notes 2008; 21:23-6
- Singhal H, Bautista DS, Tonkin KS, O'Malley FP, Tuck AB, Chambers AF, Harris JF. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin Cancer Res 1997; 3:605-11; PMID:9815727
- Oates AJ, Barraclough R, Rudland PS. The identification of osteopontin as a metastasis-related gene product in a rodent mammary tumour model. Oncogene 1996; 13:97-104; PMID:8700559
- Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell 2010; 18:533-43; PMID:20412769; http://dx.doi.org/10.1016/j.devcel.2010.02.013
- Shevde LA, Samant RS, Goldberg SF, Sikaneta T, Alessandrini A, Donahue HJ, Mauger DT, Welch DR. Suppression of human melanoma metastasis by the metastasis suppressor gene, BRMS1. Exp Cell Res 2002; 273:229-39; PMID:11822878; http://dx.doi.org/10.1006/excr.2001.5452
- Seraj MJ, Harding MA, Gildea JJ, Welch DR, Theodorescu D. The relationship of BRMS1 and RhoGDI2 gene expression to metastatic potential in lineage related human bladder cancer cell lines. Clin Exp Metast 2000; 18:519-25; PMID:11592309; http://dx.doi.org/10.1023/A:1011819621859
- Smith PW, Liu Y, Siefert SA, Moskaluk CA, Petroni GR, Jones DR. Breast cancer metastasis suppressor 1 (BRMS1) suppresses metastasis and correlates with improved patient survival in non-small cell lung cancer. Cancer Lett 2009; 276:196-203; PMID:19111386; http://dx.doi.org/10.1016/j.canlet.2008.11.024
- Saunders MM, Seraj MJ, Li Z, Zhou Z, Winter CR, Welch DR, Donahue HJ. Breast cancer metastatic potential correlates with a breakdown in homospecific and heterospecific gap junctional intercellular communication. Cancer Res 2001; 61:1765-7; PMID:11280719
- Slipicevic A, Holm R, Emilsen E, Ree Rosnes AK, Welch DR, Maelandsmo GM, Florenes VA. Cytoplasmic BRMS1 expression in malignant melanoma is associated with increased disease-free survival. BMC Cancer 2012; 12:73; PMID:22356677; http://dx.doi.org/10.1186/1471-2407-12-73
- Nagji AS, Liu Y, Stelow EB, Stukenborg GJ, Jones DR. BRMS1 transcriptional repression correlates with CpG island methylation and advanced pathological stage in non-small cell lung cancer. J Pathol 2010; 221:229-37; PMID:20455258; http://dx.doi.org/10.1002/path.2707
- Yang J, Shen Y, Liu B, Tong Y. Promoter methylation of BRMS1 correlates with smoking history and poor survival in non-small cell lung cancer patients. Lung Cancer 2011; 74:305-9; PMID:21726917; http://dx.doi.org/10.1016/j.lungcan.2011.03.002
- Lombardi G, Di Cristofano C, Capodanno A, Iorio MC, Aretini P, Isola P, Tancredi M, Collecchi P, Naccarato AG, Porta RP, et al. High level of messenger RNA for BRMS1 in primary breast carcinomas is associated with poor prognosis. Int J Cancer 2007; 120:1169-78; PMID:17163420; http://dx.doi.org/10.1002/ijc.22379
- Hicks DG, Yoder BJ, Short S, Tarr S, Prescott N, Crowe JP, Dawson AE, Budd GT, Sizemore S, Cicek M, et al. Loss of breast cancer metastasis suppressor 1 protein expression predicts reduced disease-free survival in subsets of breast cancer patients. Clin Cancer Res 2006; 12:6702-8; PMID:17121889; http://dx.doi.org/10.1158/1078-0432.CCR-06-0635
- Kim B, Nam HJ, Pyo KE, Jang MJ, Kim IS, Kim D, Boo K, Lee SH, Yoon JB, Baek SH, et al. Breast cancer metastasis suppressor 1 (BRMS1) is destabilized by the Cul3-SPOP E3 ubiquitin ligase complex. Biochem Biophys Res Commun 2011; 415:720-6; PMID:22085717; http://dx.doi.org/10.1016/j.bbrc.2011.10.154
- Sarcevic B, Mawson A, Baker RT, Sutherland RL. Regulation of the ubiquitin-conjugating enzyme hHR6A by CDK-mediated phosphorylation. EMBO J 2002; 21:2009-18; PMID:11953320; http://dx.doi.org/10.1093/emboj/21.8.2009
- Grana X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995; 11:211-9; PMID:7624138
- Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, et al. Cdk5 is essential for synaptic vesicle endocytosis. Nat Cell Biol 2003; 5:701-10; PMID:12855954; http://dx.doi.org/10.1038/ncb1020
- Davidson G, Niehrs C. Emerging links between CDK cell cycle regulators and Wnt signaling. Trends Cell Biol 2010; 20:453-60; PMID:20627573; http://dx.doi.org/10.1016/j.tcb.2010.05.002
- Chen S, Bohrer LR, Rai AN, Pan Y, Gan L, Zhou X, Bagchi A, Simon JA, Huang H. Cyclin-dependent kinases regulate epigenetic gene silencing through phosphorylation of EZH2. Nat Cell Biol 2010; 12:1108-14; PMID:20935635; http://dx.doi.org/10.1038/ncb2116
- Wei Y, Chen YH, Li LY, Lang J, Yeh SP, Shi B, Yang CC, Yang JY, Lin CY, Lai CC, et al. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat Cell Biol 2011; 13:87-94; PMID:21131960; http://dx.doi.org/10.1038/ncb2139
- Wu SC, Zhang Y. Cyclin-dependent kinase 1 (CDK1)-mediated phosphorylation of enhancer of zeste 2 (Ezh2) regulates its stability. J Biol Chem 2011; 286:28511-9; PMID:21659531; http://dx.doi.org/10.1074/jbc.M111.240515
- Tudhope SJ, Wang CC, Petrie JL, Potts L, Malcomson F, Kieswich J, Yaqoob MM, Arden C, Hampson LJ, Agius L. A novel mechanism for regulating hepatic glycogen synthesis involving serotonin and cyclin-dependent kinase-5. Diabetes 2012; 61:49-60; PMID:22106156; http://dx.doi.org/10.2337/db11-0870
- Zhao X, Feng D, Wang Q, Abdulla A, Xie XJ, Zhou J, Sun Y, Yang ES, Liu LP, Vaitheesvaran B, et al. Regulation of lipogenesis by cyclin-dependent kinase 8-mediated control of SREBP-1. J Clin Invest 2012; 122:2417-27; PMID:22684109; http://dx.doi.org/10.1172/JCI61462
- Bae DS, Cho SB, Kim YJ, Whang JD, Song SY, Park CS, Kim DS, Lee JH. Aberrant expression of cyclin D1 is associated with poor prognosis in early stage cervical cancer of the uterus. Gynecol Oncol 2001; 81:341-7; PMID:11371120; http://dx.doi.org/10.1006/gyno.2001.6196
- Bartkova J, Lukas J, Muller H, Lutzhoft D, Strauss M, Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer 1994; 57:353-61; PMID:8168995; http://dx.doi.org/10.1002/ijc.2910570311
- Gansauge S, Gansauge F, Ramadani M, Stobbe H, Rau B, Harada N, Beger HG. Overexpression of cyclin D1 in human pancreatic carcinoma is associated with poor prognosis. Cancer Res 1997; 57:1634-7; PMID:9134998
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999; 398:422-6; PMID:10201372; http://dx.doi.org/10.1038/18884
- Arsic N, Bendris N, Peter M, Begon-Pescia C, Rebouissou C, Gadea G, Bouquier N, Bibeau F, Lemmers B, Blanchard JM. A novel function for Cyclin A2: control of cell invasion via RhoA signaling. J Cell Biol 2012; 196:147-62; PMID:22232705; http://dx.doi.org/10.1083/jcb.201102085
- Liu F, Korc M. Cdk4/6 inhibition induces epithelial-mesenchymal transition and enhances invasiveness in pancreatic cancer cells. Mol Cancer Ther 2012; 11:2138-48; PMID:22869556; http://dx.doi.org/10.1158/1535-7163.MCT-12-0562
- Gunasinghe NP, Wells A, Thompson EW, Hugo HJ. Mesenchymal-epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev 2012; 31:469-78; PMID:22729277; http://dx.doi.org/10.1007/s10555-012-9377-5
- Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 2013; 19:1438-49; PMID:24202396; http://dx.doi.org/10.1038/nm.3336
- Lehn S, Tobin NP, Berglund P, Nilsson K, Sims AH, Jirstrom K, Harkonen P, Lamb R, Landberg G. Down-regulation of the oncogene cyclin D1 increases migratory capacity in breast cancer and is linked to unfavorable prognostic features. Am J Pathol 2010; 177:2886-97; PMID:20971731; http://dx.doi.org/10.2353/ajpath.2010.100303
- Tobin NP, Sims AH, Lundgren KL, Lehn S, Landberg G. Cyclin D1, Id1 and EMT in breast cancer. BMC Cancer 2011; 11:417; PMID:21955753; http://dx.doi.org/10.1186/1471-2407-11-417
- Mejlvang J, Kriajevska M, Vandewalle C, Chernova T, Sayan AE, Berx G, Mellon JK, Tulchinsky E. Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell 2007; 18:4615-24; PMID:17855508; http://dx.doi.org/10.1091/mbc.E07-05-0406
- Bae E, Sato M, Kim RJ, Kwak MK, Naka K, Gim J, Kadota M, Tang B, Flanders KC, Kim TA, et al. Definition of smad3 phosphorylation events that affect malignant and metastatic behaviors in breast cancer cells. Cancer Res 2014; 74:6139-49; PMID:25205100; http://dx.doi.org/10.1158/0008-5472.CAN-14-0803
- Rivera J, Megias D, Navas C, Bravo J. Identification of essential sequences for cellular localization in BRMS1 metastasis suppressor. PLoS One 2009; 4:e6433; PMID:19649328; http://dx.doi.org/10.1371/journal.pone.0006433
- Barbaro V, Testa A, Di Iorio E, Mavilio F, Pellegrini G, De Luca M. C/EBPdelta regulates cell cycle and self-renewal of human limbal stem cells. J Cell Biol 2007; 177:1037-49; PMID:17562792; http://dx.doi.org/10.1083/jcb.200703003
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72:8463-71; PMID:9765382
