Neuroblastoma, a malignant tumor of the sympathetic nervous system, is the most common extracranial solid cancer in children. The clinical courses of the disease are remarkably heterogeneous: While roughly half of the tumors may regress spontaneously or after limited therapy, the other half is considered to be high-risk disease, which is fatal in the majority of patients despite intensive multimodal treatment. The genetic etiology and the molecular mechanisms underlying these contrasting phenotypes have remained poorly understood to date. Genomic amplification of the proto-oncogene MYCN, which has been identified in neuroblastoma more than 30 years ago,Citation1 is strongly associated with unfavorable patient outcome, and has therefore been established as a robust marker for defining high-risk disease. However, MYCN amplification is found in only 40% of high-risk neuroblastoma, leaving the question which molecular events may drive the remaining 60% of this disease subtype. Over recent years, several massive parallel sequencing studies on neuroblastoma have been conducted, which consistently reported a low frequency of recurrent mutations in this malignancy.Citation2-5 Most notably, inactivating mutations of the chromatin-remodeling gene ATRX had been identified in a small subset of high-risk disease in mutually exclusive fashion with MYCN amplifications,Citation2,3 and were found to be associated with activation of the alternative lengthening of telomeres (ALT) pathway.Citation2
To gain insight into the genetic etiology of neuroblastoma, we recently performed whole-genome sequencing of 56 primary neuroblastomas, and discovered recurrent genomic rearrangements in a 50 kb region proximal of the telomerase reverse transcriptase (TERT) gene on chromosome 5p15.33 in 12 of these cases.Citation6 The rearrangements affected high-risk tumors only and occurred in mutually exclusive fashion with MYCN amplification and ATRX mutations. In an extended cohort of 217 neuroblastomas, we observed 28 TERT rearrangements (13%), 27 of which were detected in the high-risk subgroup (24%). Thus, TERT rearrangements constitute the second most frequent gene-specific mutation in neuroblastoma and define a subgroup of high-risk disease.
We found that TERT rearrangements were consistently associated with massive transcriptional upregulation of TERT and adjacent genes distal of the breakpoints, and with substantial epigenetic remodeling of this genomic region. In fact, we detected juxtaposition of super-enhancer elements in close proximity to the TERT locus in all cases investigated, suggesting that genomic repositioning of regulatory elements might be the cause of TERT induction. We also observed strong upregulation of TERT expression in MYCN-amplified tumors, whereas high-risk neuroblastoma without TERT or MYCN alterations had low TERT mRNA levels, lacked telomerase activity and were characterized by activation of the ALT pathway. By contrast, we did not find any evidence for active telomere maintenance mechanisms in low-risk tumors.
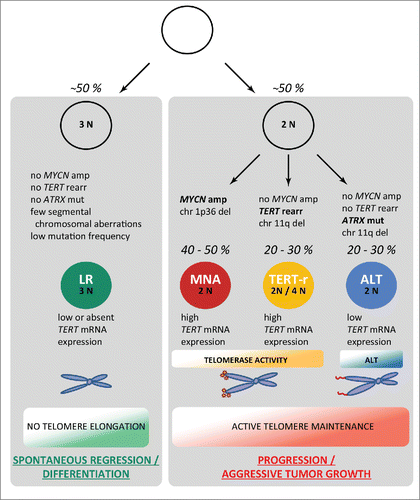
Our data fundamentally advance our understanding of the genetic basis and molecular pathogenesis of neuroblastoma (): The majority of high-risk neuroblastomas are affected by genetic alterations of either MYCN, TERT or ATRX, all of which converge on activation of telomere lengthening mechanisms. Telomeres are not replicated sufficiently in most somatic cells and thus get shortened with each cell division, eventually leading to replicative senescence or cell death. By contrast, cancer cells acquire the capability of maintaining their telomeres, thereby providing the cells with an infinite proliferation capacity. This property may be either conferred by activation of the enzyme telomerase, the catalytic subunit of which is encoded by TERT, or by alternative lengthening of telomeres, which is based on a DNA repair/homologous recombination mechanism. In neuroblastoma, the most aggressive form of the disease appears to be defined by activation of telomerase, which is induced by either TERT rearrangements or MYCN amplification. In the vast majority of the remaining high-risk tumors the ALT pathway is activated, which is associated with ATRX mutations in a fraction of this subtype. By contrast, low-risk tumors seem to lack any telomere maintenance mechanisms, which may explain their inability to gain immortal proliferation capacity and consecutively their potential for spontaneous regression. Our conclusions are strongly supported by another recent study that independently reported on TERT-rearrangements, ATRX mutations, and MYCN amplifications at similar frequencies in high-risk neuroblastomas.Citation7
Figure 1. A model for neuroblastoma pathogenesis based on recurrent genomic alterations (modified after ref. 6). High-risk neuroblastoma differs from low-risk tumors (LR) by active mechanisms of telomere lengthening: (i) via telomerase activation as a result of either TERT rearrangements (TERT-r) or amplified MYCN (MNA), or (ii) by alternative lengthening of telomeres (ALT). 2N, near-diploid karyotype; 3N, near-triploid karyotype; 4N, near-tetraploid karyotype; amp, amplification; rearr, rearrangement; mut, mutation; del, deletion.
Our findings provide new entry points for improved diagnostics and therapy of neuroblastoma patients. Diagnostic assessment of genomic alterations of MYCN, TERT and ATRX may facilitate the delineation of distinct prognostic patient subgroups. More importantly, advances in the development of telomerase inhibitors and their evaluation in clinical trials open up an opportunity to establish biomarker-based effective treatment strategies for patients with the deadliest subtype of this disease.
References
- Schwab M, et al. Nature 1983; 305:245–8; PMID:6888561
- Cheung NK, et al. JAMA 2012; 307:1062–71; PMID:22416102; http://dx.doi.org/10.1001/jama.2012.228
- Molenaar JJ, et al. Nature 2012; 483:589–93; PMID:22367537; http://dx.doi.org/10.1038/nature10910
- Pugh TJ, et al. Nat Genet 2013; 45:279–84; PMID:23334666; http://dx.doi.org/10.1038/ng.2529
- Sausen M, et al. Nat Genet 2013; 45:12–7; PMID:23202128; http://dx.doi.org/10.1038/ng.2493
- Peifer M, et al. Nature 2015; 526:700–4; PMID:26466568; http://dx.doi.org/10.1038/nature14980
- Valentijn LJ, et al. Nat Genet 2015; 47:1411–14; PMID:26523776; http://dx.doi.org/10.1038/ng.3438
