ABSTRACT
Entry into mitosis requires the phosphorylation of multiple substrates by cyclin B-Cdk1, while exit from mitosis requires their dephosphorylation, which depends largely on the phosphatase PP2A in complex with its B55 regulatory subunit (Tws in Drosophila). At mitotic entry, cyclin B-Cdk1 activates the Greatwall kinase, which phosphorylates Endosulfine proteins, thereby activating their ability to inhibit PP2A-B55 competitively. The inhibition of PP2A-B55 at mitotic entry facilitates the accumulation of phosphorylated Cdk1 substrates. The coordination of these enzymes involves major changes in their localization. In interphase, Gwl is nuclear while PP2A-B55 is cytoplasmic. We recently showed that Gwl suddenly relocalizes from the nucleus to the cytoplasm in prophase, before nuclear envelope breakdown and that this controlled localization of Gwl is required for its function. We and others have shown that phosphorylation of Gwl by cyclin B-Cdk1 at multiple sites is required for its nuclear exclusion, but the precise mechanisms remained unclear. In addition, how Gwl returns to its nuclear localization was not explored. Here we show that cyclin B-Cdk1 directly inactivates a Nuclear Localization Signal in the central region of Gwl. This phosphorylation facilitates the cytoplasmic retention of Gwl, which is exported to the cytoplasm in a Crm1-dependent manner. In addition, we show that PP2A-Tws promotes the return of Gwl to its nuclear localization during cytokinesis. Our results indicate that the cyclic changes in Gwl localization at mitotic entry and exit are directly regulated by the antagonistic cyclin B-Cdk1 and PP2A-Tws enzymes.
KEYWORDS:
Introduction
Mitotic progression relies on the coordinated activities of several kinases and their counteracting phosphatases. Cyclin B-Cdk1 triggers mitotic events such as DNA condensation, the assembly of a mitotic spindle and nuclear envelope breakdown by phosphorylating a multitude of proteins. Cdk1 activity is opposed mainly by PP2A in complex with its B55 type regulatory subunits (Tws in Drosophila).Citation1-3 Upon mitotic entry, PP2A-B55 is inhibited to promote the phosphorylated state of Cdk1 substrates. This event requires the Gwl kinase (called Mastl in mammals), which is activated at mitotic entry and subsequently phosphorylates the small protein α-Endosulfine (also known as Ensa or Endos) and its homolog Arpp19.Citation4-6 Once phosphorylated by Gwl, α-Endosulfine/Arpp19 binds and inhibits PP2A-B55 competitively.Citation4,5,7,8 In Xenopus egg cycling extracts, Gwl depletion completely prevents mitotic entry.Citation9 In Drosophila, although gwl null mutant neuroblasts enter mitosis, nuclear envelope breakdown (NEBD) is delayed and defects in chromosomes condensation, congression and segregation are frequent.Citation10,11 Similar phenotypes have been reported in Mastl-depleted human cells and in knockout mouse embryonic fibroblasts.Citation12-14 These data indicate that Gwl-dependent inhibition of PP2A-B55 is required for timely and faithful mitotic progression. In addition, Gwl activity has also been shown to be essential for meiotic maturation and meiotic arrests in flies and in vertebrates.Citation11,15-17
The mechanisms regulating Gwl are not completely understood. Gwl activation requires its phosphorylation by cyclin B-Cdk1 in the T-loop of the kinase domain.Citation9 It also requires phosphorylation in its C-terminal tail, probably by an intramolecular reaction, which is thought to induce an interaction between the C-tail and the N-terminal part of the kinase domain to stabilize its active conformation.Citation18,19 The Hsp90 and Cdc37 chaperones are also required to stabilize Gwl.Citation20 Gwl contains a long region which splits its kinase domain into N-terminal and C-terminal sub-domains, and contributes to its regulation.Citation10 Although this region is conserved in all Gwl orthologs, its amino-acid sequence is poorly conserved. No specific sequence in this middle region is essential for Gwl kinase activity.Citation19 We recently showed that the middle region of Drosophila Gwl is required for the nuclear localization of the protein via 2 Nuclear Localization Signals (NLS).Citation21 Mammalian and Xenopus Gwl were also found to contain at least one NLS in their central region.Citation14,20 In all these organisms, the NLS-dependent nuclear localization of Gwl during interphase promotes its function and mitotic progression.Citation14,20,21 The nuclear concentration of Gwl is thought to facilitate its activation by cyclin B-Cdk1 which concentrates in the nucleus while it becomes activated during mitotic entry, before NEBD.Citation14,21,22 In addition, it was hypothesized that the coordinated activation of cyclin B-Cdk1 and Gwl in the nucleus could be facilitated by the fact that they are effectively sequestrated from PP2A-B55, which is mostly cytoplasmic.Citation21-23
From its nuclear localization in interphase, Drosophila Gwl suddenly relocalizes to the cytoplasm in prophase (before NEBD), until it becomes excluded from the nucleus.Citation21 A similar dynamics was observed for Mastl in human cells.Citation14 This relocalization of Gwl is important for mitotic progression, presumably because Gwl needs to access the cytoplasmic compartment to efficiently phosphorylate Endos and antagonize PP2A-B55 in the cytoplasm before NEBD to avoid subsequent mitotic collapse.Citation14,21 We have shown that phosphorylation by Polo and Cdk1 in the middle region of Drosophila Gwl promotes its cytoplasmic localization in prophase.Citation21 Phosphorylation by Polo promotes the association of Gwl with 14-3-3ε leading to its cytoplasmic retention.Citation21 Interestingly, Rim15, the budding yeast ortholog of Gwl, was shown to be regulated in a similar fashion.Citation24 The Sch9 and Pho80-Pho85 kinases phosphorylate Rim15 to allow its interaction with Bmh1 and Bmh2 (yeast 14-3-3 proteins), which promotes Rim15 cytoplasmic retention and thus prevents G0 entry.Citation24,25 In addition, in flies and in human cells, the relocalization of Gwl from the nucleus to the cytoplasm requires its phosphorylation by cyclin B-Cdk1.Citation14,21 However, the precise mechanism by which this is achieved remains unclear. In mouse cells, mutation of known Cdk1 activation sites in the T-loop of Maslt, or a kinase-dead mutation in Mastl, prevents its cytoplasmic relocalization in prophase.Citation14 Therefore, the activation of Mastl by Cdk1 is required for its ability to relocalize in prophase. However, mutation of 6 Cdk1 sites in the central region of Drosophila Gwl, outside its kinase domain, also prevents its relocalization, suggesting that another mechanism is at play.Citation21
How Gwl is regulated at mitotic exit is poorly understood. It has been shown that PP2A-B55 itself is responsible for the inactivation of its competitive inhibitor Endos, which is bound to PP2A-B55 with extremely high affinity but is dephosphorylated inefficiently.Citation7 It is only when PP2A-B55 has freed itself from this inhibition that it can effectively target its other substrates in late mitotic exit.Citation7,26 In principle, as long as Gwl is active, it continuously generates phosphorylated Endos, thus keeping PP2A-B55 inhibited. As Gwl activation and nucleo-cytoplasmic relocalization at mitotic entry requires phosphorylation, it is presumed that its inactivation and return to the nuclear compartment require phosphatase activity. Gwl has been observed to associate with PP2A-B55, but the reaction taking place in this complex is unknown.Citation27,28 PP2A can dephosphorylate and inactivate Xenopus Gwl in vitro.Citation28 Furthermore, simultaneous RNAi silencing of B55α and B55δ results in an increased phosphorylation level of Mastl at a T-loop site in HeLa cells.Citation29 However, it appears unlikely that PP2A-B55 could be responsible for the initial inactivation of Gwl since PP2A-B55 is antagonized by Gwl activity. Thus, the specific contribution of PP2A-B55 in the regulation of Gwl is unclear.
Here, we studied the role of Cdk1 in promoting the cytoplasmic relocalization of Gwl in prophase and the regulation of Gwl by PP2A-Tws in Drosophila. We show that phosphorylation at Thr562, a Cdk1 site immediately adjacent to an NLS in the central region of Gwl, is responsible for the inactivation of its nuclear import via this NLS in prophase. Moreover, we show that PP2A-Tws activity is required for timely nuclear relocalization of Gwl during mitotic exit, and that Thr562 is important for this control. Our findings demonstrate the direct and opposite roles of cyclin B-Cdk1 and PP2A-Tws in controlling the localization of Gwl in the cell cycle.
Results
Gwl localization is regulated by nuclear import and export
We previously showed that Gwl rapidly relocalizes from the nucleus to the cytoplasm in prophase.Citation21 The clear and quick exclusion of Gwl from the nucleus suggested that Gwl is either rapidly degraded specifically in the nucleus or actively exported from the nucleus to the cytoplasm. Gwl is not known to be regulated by proteolysis. Experiments in human cells showed that the nuclear relocalization of Mastl from the nucleus in prophase is inhibited by leptomycin B, an inhibitor of the nuclear export factor Crm1.Citation14 We tested if this also holds true for Drosophila Gwl using D-Mel2 cells in culture expressing Gwl-GFP. In control cells, the nuclear exclusion of Gwl-GFP is observed for approximately 5 min before NEBD. This exclusion is manifested by a uniformly darker nucleus compared to the cytoplasm (). As expected, the exclusion is abolished by treatment with leptomycin B. In this case, Gwl-GFP diffuses to the cytoplasm when the nuclear envelope breaks down and the only apparent exclusion of Gwl is in the areas occupied by condensed chromosomes. This result indicates that the nuclear exclusion of Gwl normally observed in prophase requires Crm1-dependent export of Gwl from the nucleus to the cytoplasm ().
Figure 1. The nuclear exclusion of Gwl in prophase depends on active export. (A) The nuclear exclusion of Gwl in prophase is inhibited by leptomycin B. Cells were treated with 50 μM leptopmycin B or ethanol alone and filmed immediately. (B) Identification of an NES motif in Gwl (residues 553–558). This motif was predicted as a highly probable NES using ValidNESs.Citation36 (C) Mutation of the NES abolished the nuclear exclusion of Gwl in prophase. NESm = L556A, I558A. Time zero (T0) was defined as the time when an increase in cytoplasmic Gwl is first noticed. Scale bars: 5 μm.
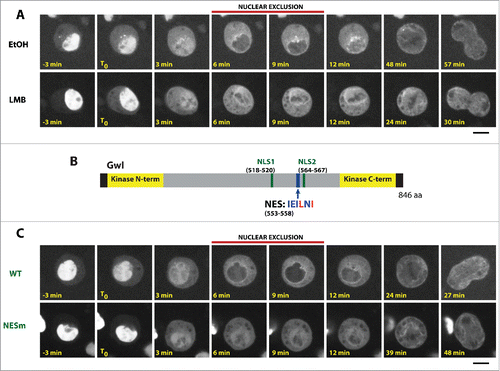
We identified a putative nuclear export sequence (NES) in Gwl (). Mutation of this motif (L556A, I558A) abolished the nuclear exclusion of Gwl in prophase (). We previously showed that the nuclear localization of Gwl depends on 2 NLS motifs in its central region ().Citation21 Mutation of both NLSs is required for a clear cytoplasmic retention of Gwl, while mutation of a single NLS induces only a partial cytoplasmic relocalization. Mutation of the hypothetical NES could partially reduce the cytoplasmic localization of Gwl caused by mutation of its NLSs (). It was previously shown that the kinase activity of Mastl is required for its nuclear exclusion in prophase.Citation14 We obtained similar results with Drosophila Gwl. A kinase-dead mutation (K87R)Citation11 or mutation of its C-terminal auto-phosphorylation activation site (S840A)Citation19 abolished the nuclear exclusion of Gwl (Fig S1A-C). Thus, mutation of the putative NES motif could have abrogated the nuclear exclusion of Gwl if it killed its kinase activity. This was unlikely as the motif is not strictly conserved in vertebrates (data not shown). We nevertheless tested if the putative NES motif was required for Gwl kinase activity. In vitro kinase assays showed that mutation of the NES did not kill the kinase activity of Gwl (Fig S1D). These results suggest that the identified motif is a genuine active NES in Gwl.
Figure 2. Gwl shuttles between the nucleus and the cytoplasm. (A) Mutation of the NES motif partially rescues the localization defects due to mutations of NLS motifs in Gwl. The relative fluorescence intensities of the cytoplasm and nucleus were measured for the indicated versions of Gwl-GFP. Scale bar: 5 μm. (B) Fluorescence loss in photobleaching (FLIP). Irradiation of a small portion of the cytoplasm (A1) for repeated cycles (30 total) induces the bleaching of Gwl-GFP in the nucleus (measured in A2). A region A3 in a neighboring cell was measured to subtract the bleaching induced by the image acquisition (see Materials & Methods). Calculations were done for the indicated time points. Numbers are averages of multiple cells ± SEM. Note that mutation of the NES in Gwl slows down the bleaching.
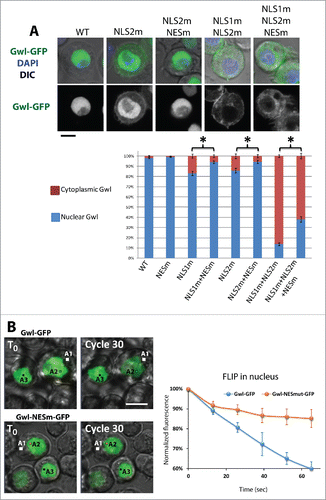
To visualize the nucleocytoplasmic shuttling of Gwl in real time, we performed fluorescence loss in photobleaching (FLIP) experiments. In cells expressing Gwl-GFP, we repeatedly photobleached a small area in the cytoplasm (A1) and measured the decrease in nuclear GFP fluorescence in a second area (A2) as a function of time. As expected for a protein shuttling between the cytoplasm and the nucleus, bleaching of the cytoplasmic Gwl-GFP in interphase cells resulted in a decrease of the Gwl-GFP signal in the nucleus (). Mutation of the NES resulted in a much slower decrease of the nuclear Gwl-GFP signal during FLIP experiments. By contrast, bleaching of nuclear Gwl-GFP resulted in a much faster decrease of nuclear GFP fluorescence and mutation of the NES had little or no effect, indicating that Gwl-GFP and Gwl-NESm-GFP diffuse at similar rates inside the nucleus (Fig. S2). We conclude that Gwl is constantly shuttling between the nucleus and the cytoplasm in interphase cells, and that it is actively exported from the nucleus via a conserved NES motif.
Phosphorylation at a Cdk1 site directly inhibits the nuclear import of Gwl
We previously showed that cyclin B-Cdk1 phosphorylates Gwl at multiple sites in its central region, and that simultaneous mutation of these residues prevents the relocalization of Gwl from the nucleus to the cytoplasm in prophase.Citation21 It has been shown that cyclin B-Cdk1 activates Gwl by phosphorylation of sites in its kinase domain.Citation9 We know that Gwl requires its own kinase activity for its nuclear exclusion (Fig. S1 andCitation14), although the underlying molecular mechanism is unknown. Therefore, cyclin B-Cdk1 is expected to promote the nuclear exclusion of Gwl by phophorylating and activating its kinase domain. Indeed, in human cells, mutation of Cdk1 sites in the kinase domain of Mastl known to control its kinase activity abolishes its nuclear exclusion in prophase.Citation14 However, the cyclin B-Cdk1 sites that we identified as required for Gwl nuclear exclusion are distinct and outside the kinase domain. We therefore investigated if Cdk1 could also contribute to regulate Gwl localization more directly, by modulating its nuclear import or export. In particular, we focused our attention on Thr562, a Cdk1 site previously detected by mass spectrometry and immediately adjacent to NLS2 in Gwl ().Citation21 We found that alanine substitution of Thr562 abolishes the nuclear exclusion of Gwl in prophase (). However, the additional mutation of NLS2 restored the capacity of Gwl-T562A to be excluded from the nucleus (). These results suggest that phosphorylation at Thr562 inactivates NLS2 and is required for the efficient relocalization of Gwl to the cytoplasm in prophase, although other mechanisms are sufficient for Gwl nuclear exclusion if NLS2 is inactivated.
Figure 3. Phosphorylation near an NLS is required for the nuclear exclusion of Gwl in prophase. (A) Thr562 (red) is a phosphorylation site immediately adjacent to NLS2 (green) in Gwl. To be compared with Gwl-WT-GFP in . (B) Alanine mutation of Thr562 abolishes the nuclear exclusion of Gwl in prophase. (C) Phosphomimetic mutations of Thr562 inactivate NLS2 and promote the cytoplasmic retention of Gwl. Scale bars: 5 μm. The relative fluorescence intensities of the cytoplasm and nucleus were measured for the indicated versions of Gwl-GFP. * p = 0.02, *** p < 1 × 10−13.
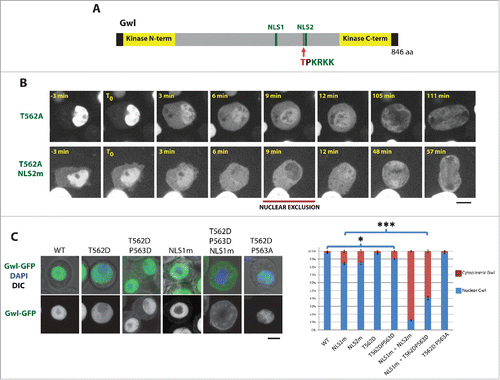
To further evaluate if phosphorylation at Thr562 could inactivate NLS2, we introduced phospho-mimetic mutations. The T562D substitution alone did not significantly abrogate the nuclear localization of Gwl. We reasoned that if a negatively charged phosphate group inactivates the NLS by a neutralization effect (NLS2 has 4 positive charges), then an aspartic acid residue may not fully mimic phosphorylation because it introduces a single negative charge instead of the 2 negative charges of a phosphate. Therefore, we decided to test the introduction of a second negative charge, with the P563D substitution. We found that Gwl-T562D-P563D was partially retained in the cytoplasm in interphase, to a similar level to Gwl-NLS2m (). The P563D mutation alone was less effective in causing cytoplasmic retention of Gwl-GFP (7 ± 3% of cytoplasmic intensity vs 13 ± 4% for T562D-P563D and 4 ± 3% for WT measured in a separate experiment from that shown in ). Because mutation of both identified NLSs is needed to efficiently prevent the concentration of Gwl in the nucleus ( andCitation21), we introduced the T562D-P563D substitutions in a background already mutated to inactivate NLS1 in Gwl. As expected, Gwl-NLS1m-T562D-P563D was strongly retained in the cytoplasm (). Altogether, our results indicate that phosphorylation of Gwl at a Cdk1 site directly inactivates an NLS and thereby contributes to promote the relocalization of Gwl from the nucleus to the cytoplasm during prophase, as the cell enters mitosis.
PP2A-Tws promotes the return of Gwl to the nucleus after mitosis
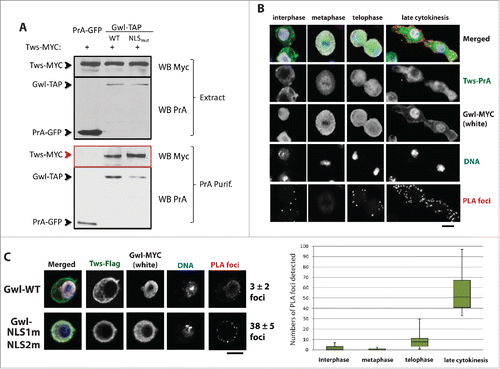
Because the nuclear localization of Gwl is negatively regulated by phosphorylation, we hypothesized that phosphatase activity could promote the return of Gwl to the nucleus after mitosis. PP2A-Tws/B55 is known to efficiently dephosphorylate Cdk1 sites. We therefore considered the possibility that PP2A-Tws could contribute to the return of Gwl to the nucleus by dephosphorylating its Cdk1 sites. Moreover, Gwl and PP2A-B55 were shown to associate in Xenopus, but the function for this interaction remained elusive.Citation28 Using co-purifications and Western blots, we found that Drosophila Gwl and Tws associate, like in Xenopus (). We used Proximal Ligation Assay (PLA) to probe the association between Gwl and Tws, allowing the detection of the complex at specific cell cycle stages and in different cellular compartments. We confirmed that Tws and Gwl associate in cells. PLA foci were most frequent in telophase and cytokinetic cells (). Interestingly, foci were almost exclusively detected in the cytoplasm. A mutant form of Gwl which is retained in the cytoplasm even in interphase (Gwl-NLS1m-NLS2m) associated more strongly with Tws in the co-purification assay, and induced more Gwl-Tws PLA foci in interphase cells (). These observations suggest that the Gwl/Tws interaction is normally prevented in interphase by the physical sequestration of these proteins. The interaction between PP2A-Tws and Gwl in the cytoplasm of telophase/cytokinetic cells is ideally poised to promote the dephosphorylation of Gwl when it returns to its nuclear localization.
Figure 4. Gwl associates with PP2A-Tws in the cytoplasm during late mitotic exit. (A) D-Mel cells were transfected with plasmids for the expression of the indicated proteins. Cells were used in Protein A-affinity purifications followed by Western blots. Tws-Myc was co-purified specifically with Gwl-PrA. Mutation of the 2 NLSs enhanced this association. (B) D-Mel cells expressing Tws-Myc and Gwl-PrA were submitted to Proximal Ligation Assay (PLA). PLA foci were visualized on a confocal microscope and quantified. PLA foci were detected mostly in telophase and during cytokinesis, in the cytoplasm and not in the nuclei. (C) Tws-Flag/Gwl-Myc PLA signals are enhanced when the 2 NLSs in Gwl are mutated. Numbers = average ± SEM
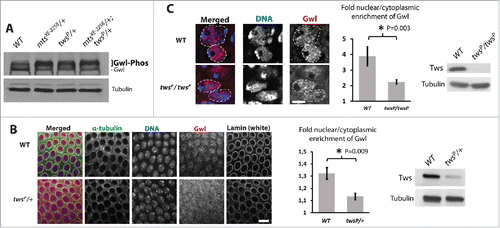
To begin to evaluate if PP2A-Tws promotes the nuclear localization of Gwl, we used Drosophila syncytial embryos, where 13 rounds of synchronous nuclear mitotic divisions occur rapidly using the maternal contribution in mRNA and proteins. In embryo extracts, Gwl is detected as a thick stack of bands corresponding to multiple phosphorylated or unphosphorylated forms.Citation11,21 We found that the slower migrating forms of Gwl were enriched at the expense of the faster migrating forms in extracts from embryos laid by mothers heterozygous for mutations in tws or mts (which encodes the catalytic subunit of PP2A-Tws) (). We used immunofluorescence to probe the localization of Gwl in syncytial embryos. In embryos from twsP/+ mothers, Gwl was slightly but significantly more cytoplasmic in interphase than in embryos from WT mothers (). Because Tws is an essential gene, we could not test the effect of a complete loss of its function in embryos. However, we were able to measure the localization of Gwl in neuroblasts of twsP/twsP larvae because these animals develop until the pupal stage using the perduring maternal contribution in Tws protein and/or mRNA. In twsP/twsP neuroblasts, the nuclear/cytoplasmic ratio of Gwl localization was lower than that in WT controls (). These results suggest that PP2A-Tws promotes the nuclear localization of Gwl in mitotically active tissues in vivo, both in syncytial embryos and in larval neuroblasts.
Figure 5. PP2A-Tws promotes the nuclear localization of Gwl in vivo. (A) Gwl is hyperphosphorylated in embryos from mothers heterozygous for mutations in mts and tws, as detected by slower-migrating forms of Gwl by Western blot. (B) Gwl is more cytoplasmic in embryos from mothers heterozygous for a mutation in tws. Left: immunofluorescence in fixed syncytial embryos. Center: quantification of the nuclear vs cytoplasmic fluorescence intensities of Gwl. Right: Western blot showing the lower levels of Tws in embryos from twsP/+ mothers. (C) Gwl is more cytoplasmic in twsP/twsP larval neuroblasts. Left: immunofluorescence in fixed larval neuroblasts. Dashed lines indicate the approximated limits of nuclear areas. Center: quantification of the nuclear vs cytoplasmic fluorescence intensities of Gwl. Right: Western blot showing the lower levels of Tws in twsP/twsP whole larvae.
To assess the contribution of PP2A-Tws to the return of Gwl to its nuclear localization during the cell cycle, we used time-lapse imaging of Gwl-GFP expressing cells in culture transfected with dsRNA against Tws. Inactivation of PP2A-Tws in these cells interferes with several events of late mitotic exit, leading to problems including an increase in anaphase bridges and central spindle defects Citation(30 and our unpublished observations). Despite these defects, most cells completed cell division and daughter cells managed to return to interphase following RNAi depletion of Tws. We measured the timing of Gwl-GFP nuclear import relative to RFP-Lamin B assembly as a marker of nuclear envelope reformation (). In these cells, Gwl-GFP became visibly enriched in daughter nuclei compared to the cytoplasm on average 11 min after RFP-Lamin B began to be recruited at the nuclear periphery. By contrast, following RNAi depletion of Tws, Gwl-GFP took on average 25 min before becoming enriched in daughter nuclei (). We conclude that PP2A-Tws activity is required for the timely return of Gwl to the nucleus at the end of cell division.
Figure 6. PP2A-Tws is required for timely return of Gwl to the nuclei during late mitotic exit. (A) Stable cell lines allowing the inducible expression of RFP-Lamin B and Gwl-GFP or Gwl-T562A-GFP were transfected with dsRNA against tws or the bacterial KAN gene. Expression was induced 3 days later and cells were filmed on day 4. The time between the initial enrichment of RFP-Lamin B at the nuclear periphery (T0) and the observation of a clearly higher concentration of Gwl-GFP in daughter nuclei compared to the cytoplasm (red squares) was measured. Scale bars: 5 μm. (B) Quantification of measurements done as in (A). The delay in Gwl import caused by the depletion of Tws depends on Thr562. Error bars: SEM. (C) Western blots for the indicated proteins under the conditions used.
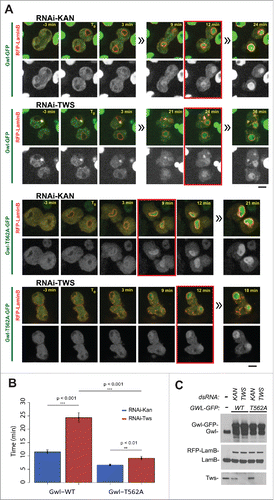
We reported above that the phosphorylation of Gwl at Thr562, near NLS2, was required for the nuclear exclusion of Gwl in prophase. Thr562 is a verified Cdk1 site.Citation21 Because PP2A-Tws efficiently dephosphorylates Cdk1 sites, we hypothesized that it could promote the return of Gwl to the nucleus by dephosphorylating Thr562. Gwl-GFP became enriched in the nuclei of daughter cells around 11 min after RFP-Lamin B recruitment. By comparison, we found that the T562A mutation advanced the return of Gwl to the nucleus by approximately 5 minutes (). Conversely, the phosphomimetic substitutions T562D-P563D dramatically delayed the return of Gwl to the nuclei. Gwl-T562D-P563D-GFP became enriched in nuclei only between 1 and 2 hours after RFP-Lamin B recruitment (Figs. S3 and S4). We conclude that dephosphorylation of Thr562 controls the reactivation of the immediately adjacent NLS2 in Gwl, and that this mechanism is required for the efficient re-import of Gwl during mitotic exit. The 2 min delay in the import of Gwl-T562A-GFP observed upon depletion of Tws suggests that PP2A-Tws may also promote the import of Gwl by dephosphorylating additional sites.
Discussion
Direct control of Gwl localization by reversible phosphorylation
Our results indicate that cyclin B-Cdk1 directly and negatively regulates the nuclear import of Gwl by phosphorylating the central region of Gwl. This mechanism is distinct from the previously shown activation of Gwl kinase by cyclin B-Cdk1, in which sites in the N-terminal part of the kinase domain of Gwl are phosphorylated. Phosphorylation at Thr562 was found to be particularly important for the inactivation of NLS2 and the cytoplasmic retention of Drosophila Gwl in prophase. Our results also show that conversely, PP2A-Tws plays a role in promoting the import of Gwl to daughter nuclei at the end of cell division, and suggest that Thr562 is a critical site in this process, although possibly not the only one. Moreover, while the inactivation of an NLS is essential for the efficient relocalization of Gwl from the nucleus to the cytoplasm in prophase, it is not sufficient. This relocalization also requires Crm1-dependent nuclear export. We report the identification of a motif in Gwl predicted to function as an NES, the mutation of which abolishes the nuclear exclusion of Gwl.
The central region of Gwl serves as a regulatory platform for its nuclear localization. It contains at least 2 NLSs and one NES, and multiple phosphorylation sites, all clustered in a small sequence segment. While we have uncovered the regulation of NLS2 by Cdk1 and PP2A-Tws, and at least one major phosphorylation site involved, the mechanisms controlling Gwl localization remain incompletely understood. Consistent with results obtained for human Mastl,Citation14 we found that kinase activity of Gwl is required for its nuclear exclusion. Intramolecular auto-activation of Gwl in its C-tail is thought to induce a conformational change that locks Gwl in its active state.Citation18,19 It is possible that this active conformation of Gwl that would normally be induced in prophase hides an NLS, or exposes the NES or phosphorylation sites, and thus promotes the nuclear exclusion of Gwl as a result. Further studies would be required to discriminate between possible mechanisms. In addition, our previous study indicated that phosphorylation of Gwl by Polo kinase and 14-3-3e binding also promote the cytoplasmic localization of Gwl.Citation21 Since NLS1 is sufficient for a significant nuclear enrichment of Gwl in interphase (), its inactivation is likely required for the exclusion of Gwl from the nucleus in prophase seen with the Gwl-T562A, NLS2m (). However, the role of Polo kinase in regulating the localization of Gwl does not appear to be conserved in humans as inhibition of Plk1 does not prevent the relocalization of Mastl in prophase.Citation14 Subtle mechanistic differences between species are to be expected since the central region of Gwl is poorly conserved at the sequence level. However, general principles of regulation are likely conserved. Human Mastl and Xenopus Gwl both contain at least one NLS and multiple phosphorylation sites in their central region.Citation14,19,20 It would be interesting to test if phosphorylation of Mastl by Cdk1 can directly inactivate an NLS motif, or if it promotes Mastl nuclear exclusion solely by activating its kinase domain. The multiple levels of regulation ensuring that Gwl is rapidly and effectively exported from the nucleus and retained in the cytoplasm before nuclear envelope breakdown suggest that considerable selective pressure has been acting during evolution to ensure that this process is coupled to cell division. In fact, a failure in Gwl nuclear exclusion in prophase is known to result in mitotic defects similar to a complete loss of Gwl function.Citation14,21 The relocalization of Gwl to the cytoplasm in prophase is thought to be necessary for the efficient phosphorylation of Endos and the ensuing inhibition of PP2A-B55. It is proposed that if PP2A-B55 is not inhibited in the cytoplasm before NEBD, it immediately dephosphorylates cyclin B-Cdk1 sites on the nuclear substrates that become exposed at NEBD.Citation14,22 These substrates are likely to include important factors for chromosome condensation and kinetochore function that must remain phosphorylated until later in mitosis. The specific identity of these substrates remains to be determined.
It has been shown that the Gwl-Endos system imposes a delay between sister chromatid segregation (which is triggered by APCCdc20 activation) and central spindle constriction (which is controlled by PP2A-B55).Citation26 Goldberg and colleagues have shown that phosphorylated Endos is a substrate of PP2A-B55 with an extremely high affinity for PP2A-B55 and which is dephosphorylated very slowly by PP2A-B55.Citation7 According to their “unfair competition” model, PP2A-B55 cannot efficiently dephosphorylate its other substrates as long as there is a stoichiometric excess of phosphorylated Endos in the cell. Consistent with it, our PLA results indicate that the association between PP2A-Tws and Gwl increases dramatically only after anaphase, even though both partners are present in the cytoplasm starting in prophase. Our results indicate that PP2A-Tws promotes the re-entry of Gwl in the newly born nuclei by the direct dephosphorylation of sites including Thr562 near an NLS. The fact that the T562A mutation advances the time of Gwl import in daughter nuclei suggests that PP2A-Tws is not fully reactivated until nuclear envelope re-formation, also in agreement with the proposed model.
Phosphatases controlling Gwl and Endos
The idea that Gwl could be subject to dephosphorylation by PP2A-B55 during the cell cycle was tantalizing already based on the fact that Gwl is a target of Cdk1 and on evidence indicating that PP2A-B55 efficiently dephosphorylates Cdk1 substrates.Citation1,3 Experimental evidence also suggested that PP2A-B55 can dephosphorylate Gwl, including at Thr194, a T-loop activation site in humans.Citation28,29 The physical sequestrations of Gwl and PP2A-B55 in the nucleus and the cytoplasm, respectively, is thought to facilitate Gwl activation by cyclin B-Cdk1 in the nucleus during prophase, without futile dephosphorylation by PP2A-B55.Citation21,22 Even if PP2A-B55 is capable of dephosphorylating Cdk1 sites in Gwl required for its kinase activity, it is unlikely to contribute to the initial inactivation of Gwl during mitotic exit. The reason is that for PP2A-B55 to become active again and begin to process Gwl, it must dephosphorylate Endos, but as long as Gwl is active, it should regenerate phosphorylated Endos, keeping PP2A-B55 inhibited. While this paper was in revision, a report by Heim and colleagues appeared, showing that PP1 is responsible for the initial inactivation of Gwl in Xenopus egg extracts, by dephosphorylating the auto-activation site in the C-terminal tail of Gwl.Citation31 Their results also suggest that this initial inactivation of Gwl allows reactivation of PP2A-B55 which can then contribute to dephosphorylate Gwl at other sites, consistent with our findings. Although clearly required for the rapid re-entry of Gwl to nuclei during mitotic exit, PP2A-B55 is unlikely to be the sole phosphatase competent for this function because Gwl ultimately returns to the nucleus when Tws is depleted, albeit with a delay. Future studies could identify contributions of other phosphatases in the regulation of Gwl.
Materials and methods
Cell culture and fly husbandry
D-Mel2 (D-Mel) were cultured in Express Five medium (Invitrogen). Stable cell lines expressing pMT-Gwl-GFP (WT and mutants) were generated as described.Citation32 Briefly, selection was based on resistance to 20 μg/ml blasticidin. Inducible pMT-based vectors contained the blasticidin resistance gene, while pAC5-based vectors were co-transfected with the pCoBast which confers cells the blasticidin resistance. Stable cultures obtained were also sorted by flow cytometry to obtain more homogeneous expression levels of Gwl-GFP and RFP-Lamin B. Flies were maintained at 25°C on standard food. Oregon R was used as wild type strain. twsP (twsJ11C8) carrying a TM6B balancer chromosome was from David Glover. mtsXE-2258 carrying a CyO balancer chromosome was from Bloomington stock center.
Plasmids
All Drosophila cells expression vectors were generated in the Gateway system (Invitrogen). Coding sequences were first cloned into the pDONR221λ entry vector. They were then recombined with destination vectors to generate the following expression plasmids: pMT-Gwl-GFP, pMT-Gwl-TAP, pAC5-Gwl-Myc, pMT-Tws-TAP, pAC5-Tws-Myc, pAC5-Tws-Flag, pAC5-PrA-GFP, pMT-RFP-Lamin B as well as all related mutants. All mutants were generated by QuickChange (Agilent Technologies) in entry clones. The Gwl and Tws coding sequences used correspond to their longest isoforms annotated in Flybase.
Immunofluorescence and western blotting
For immunofluorescence in embryos and in cells, we used protocols previously described.Citation32 Briefly, cells were fixed with 4% formaldehyde for 10 min, and permeabilized and blocked in Phosphate Buffer Saline (PBS) containing 0.1% Triton X-100 and 1% BSA (PBSTB). Cells were incubated with primary antibody diluted in PBSTB for 1 h at room temperature or O/N at 4 degree, washed 3 times in PBSTB, and incubated with secondary antibodies for 1 h to 2 h at room temperature. Cells were washed several times in PBSTB and once in PBS, before being mounted in Vectashield medium containing DAPI. Zero to 3 h old embryos were dechorionated in 50% bleach before fixation using formaldehyde and methanol. Embryos were then rehydrated, permeabilized, blocked and incubated with antibodies. DAPI was added in the secondary antibodies solution and embryos were mounted in Vectashield. Immunostaining of neuroblasts was performed as described.Citation33 Brains were dissected from the third instar larvae 6 days after laying. Antibodies used for immunofluorescence and Western blotting are: rabbit IgG (for PrA in TAP tag; MP Biochemicals) and anti-Gwl (custom-made against full-length Gwl by Genscript), anti-Myc 9E10 (Santa Cruz), M2 anti-Flag (Sigma). Secondary antibodies were coupled to Alexa-488 (1:200; Invitrogen), Texas red (1:200; Invitrogen) or Peroxidase (Jackson). DNA was marked with DAPI. In , the cytoplasmic and nuclear fluorescence of Gwl was measured by drawing boxes in ImageJ for multiple cells taken randomly. Error bars: Standard error of the mean. Asterisks: *p < 0.05, ** p < 0.01 and *** p < 0.001 after Student t-test.
Microscopy
Fixed cell images were acquired on a Laser scanning confocal microscope LSM 510 Meta (Zeiss), using a 100X oil objective. FLIP experiments were performed using a LSM700 laser scanning confocal microscope (Zeiss) with a Plan Apochromat 63× objective on cells either expressing Gwl-GFP or Gwl-NES-GFP. Expression of Gwl-GFP and Gwl-NESm-GFP was induced by adding 300 μM CuSO4 24 hours before the experiment. After an initial image acquisition, 30 cycles of photobleaching and image acquisitions were performed. In each cycle, a small region of interest (A1) from the cytoplasm was photo-bleached for 1 second (20 iterations) using full laser power, followed by 1 image acquisition. FLIP was analyzed by measuring the mean fluorescence in a ROI of the nucleus of the bleached cell (A2) prior to photobleaching and in every frames of the experiment using the ImageJ software (National Institutes of Health). Similarly, a ROI from a non-bleached neighbor cell (A3) was measured to determine the photobleaching induced during image acquisition. The FLIP data were calculated as normalized fluorescence as publishedCitation34: normalized fluorescence = ( F(A2tn) / F(A2t0) ) * ( F(A3t0) / F(A3tn) ), where F(A2tn) and F(A2t0) are the mean fluorescence of the ROI A2 at time n and at time 0 (prior to bleaching), respectively, and F(A3tn) and F(A3t0) in the ROI A3. The background fluorescence was negligible in our measurement.
All other live cell imaging was acquired on a Spinning-Disk confocal system (Yokogawa CSU-X1 5000) mounted on a fluorescence microscope (Zeiss Axio Observer.Z1) using an Axiocam 506 mono camera (Zeiss) and a 63X oil objective. For images shown in , S1 and S3 a binning of 2 × 2 was applied. For images shown in , a binning of 5 × 5 was applied. No pixel interpolation was applied.
Affinity purifications
Protein A affinity purifications from cells were carried out essentially as described.Citation35 Briefly, pelleted cells from confluent 75 cm2 flasks were resuspended in approximately 10 volumes of lysis buffer and passed through a needle several times using a syringe. Lysates were clarified by centrifugation for 15 min at 14,000 rpm in a tabletop centrifuge. Supernatants were incubated with 25 μl of IgG-conjugated DynaBeads (Invitrogen) for 1 to 2 hours and washed with lysis buffer 4 to 5 times for 5 min. Purification products were eluted by heating at 95°C for 2 min in 2x SDS-PAGE Laemmli buffer (Sigma) and analyzed by Western blotting.
Kinase assay
TAP tagged Gwl WT, Kinase-Dead, NLSm and NESm were purified from D-Mel2 cells by affinity to the PrA tag as described above and subsequently used to phosphorylate GST-Endos obtained from bacterial expression. Reactions were performed in kinase buffer (20 mM K-HEPESpH 7.5, 2 mM MgCl2, 1 mM DTT, 1 μM ATP, 1 μCi 32P-γ-ATP) at 30°C for 20 min with agitation and stopped by addition of 2x Laemmli buffer and heating at 95°C for 5 minutes. Products were resolved by SDS-PAGE and transferred onto nitrocellulose membrane for autoradiography.
Proximal ligation assay
For PLA assay, we used the Duolink starter kit from Sigma and according to the protocol of the manufacturer. Antibodies used were mouse anti-Myc 9E19 (Sigma) and rabbit IgG (MP Biochemicals) () or mouse anti-Flag M2 (Sigma) and rabbit anti-Myc (Santa Cruz) (). The plus probe was anti-mouse and the minus probe was anti-rabbit. Cells were incubated with secondary antibodies during the PLA amplification step. As controls, we ensured that PLA signals were not observed in cells expressing only one tagged protein.
RNA interference
dsRNAs corresponding to the Tws coding sequence between bp 333 and 735 or to the bacterial kanamycin resistance gene (as negative control) were synthesized using the T7 Ribomax kit (Promega) from PCR products generated with T7 sequence-containing primers (Forward: TAATACGACTCACTATAGGGAGATCCTGCCTCAAAAGCC; Reverse: TAATACGACTCACTATAGGGAGAGAAGGTCTCCTGATCCGA). The day before RNAi treatment, 1,000,000 cells stably transfected with copper-inducible Gwl-GFP (wild type and mutants) and RFP-Lamin B were seeded in 2 ml of medium in 6-well plates. The day of RNAi treatment, the 2 ml of medium was replaced with a mix of 20 μg of dsRNA and 20 μl Transfast (Promega) in 1 ml of medium. The expression of Gwl-GFP wild type or mutants and RFP-Lamin B was induced on the 3rd day post-transfection, and the cells were analyzed on the 4th day.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
1127476_Supplemental_Material.zip
Download Zip (4.4 MB)Funding
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) to VA and GE. PW held a studentship from the Fonds de recherche du Québec – Santé (FRQS). VA holds a New Investigator salary award from the CIHR. GE holds the Canada Research Chair in Vesicle Transport and Cell Signaling. IRIC is supported in part by the Canada Foundation for Innovation and the FRQS.
References
- Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol Biol Cell 2009; 20:4777-89; PMID:19793917; http://dx.doi.org/10.1091/mbc.E09-07-0643
- Mayer-Jaekel RE, Ohkura H, Ferrigno P, Andjelkovic N, Shiomi K, Uemura T, Glover DM, Hemmings BA. Drosophila mutants in the 55 kDa regulatory subunit of protein phosphatase 2A show strongly reduced ability to dephosphorylate substrates of p34cdc2. J Cell Sci 1994; 107 ( Pt 9):2609-16; PMID:7844174
- Mochida S, Ikeo S, Gannon J, Hunt T. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. Embo J 2009; 28:2777-85; PMID:19696736; http://dx.doi.org/10.1038/emboj.2009.238
- Mochida S, Maslen SL, Skehel M, Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 2010; 330:1670-3; PMID:21164013; http://dx.doi.org/10.1126/science.1195689
- Gharbi-Ayachi A, Labbe JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 2010; 330:1673-7; PMID:21164014; http://dx.doi.org/10.1126/science.1197048
- Rangone H, Wegel E, Gatt MK, Yeung E, Flowers A, Debski J, Dadlez M, Janssens V, Carpenter AT, Glover DM. Suppression of scant identifies Endos as a substrate of greatwall kinase and a negative regulator of protein phosphatase 2A in mitosis. PLoS Genet 2011; 7:e1002225; PMID:21852956; http://dx.doi.org/10.1371/journal.pgen.1002225
- Williams BC, Filter JJ, Blake-Hodek KA, Wadzinski BE, Fuda NJ, Shalloway D, Goldberg ML. Greatwall-phosphorylated Endosulfine is both an inhibitor and a substrate of PP2A-B55 heterotrimers. Elife 2014; 3:e01695; PMID:24618897
- Mochida S. Regulation of α-endosulfine, an inhibitor of protein phosphatase 2A, by multisite phosphorylation. FEBS J 2014; 281:1159-69; PMID:24354984; http://dx.doi.org/10.1111/febs.12685
- Yu J, Zhao Y, Li Z, Galas S, Goldberg ML. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell 2006; 22:83-91; PMID:16600872; http://dx.doi.org/10.1016/j.molcel.2006.02.022
- Yu J, Fleming SL, Williams B, Williams EV, Li Z, Somma P, Rieder CL, Goldberg ML. Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J Cell Biol 2004; 164:487-92; PMID:14970188; http://dx.doi.org/10.1083/jcb.200310059
- Archambault V, Zhao X, White-Cooper H, Carpenter AT, Glover DM. Mutations in Drosophila Greatwall/Scant Reveal Its Roles in Mitosis and Meiosis and Interdependence with Polo Kinase. PLoS Genet 2007; 3:e200; PMID:17997611; http://dx.doi.org/10.1371/journal.pgen.0030200
- Burgess A, Vigneron S, Brioudes E, Labbe JC, Lorca T, Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci U S A 2010; 107:12564-9; PMID:20538976; http://dx.doi.org/10.1073/pnas.0914191107
- Voets E, Wolthuis RM. MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle 2010; 9:3591-601; PMID:20818157; http://dx.doi.org/10.4161/cc.9.17.12832
- Alvarez-Fernandez M, Sanchez-Martinez R, Sanz-Castillo B, Gan PP, Sanz-Flores M, Trakala M, Ruiz-Torres M, Lorca T, Castro A, Malumbres M. Greatwall is essential to prevent mitotic collapse after nuclear envelope breakdown in mammals. Proc Natl Acad Sci U S A 2013; 110:17374-9; PMID:24101512; http://dx.doi.org/10.1073/pnas.1310745110
- Kim MY, Bucciarelli E, Morton DG, Williams BC, Blake-Hodek K, Pellacani C, Von Stetina JR, Hu X, Somma MP, Drummond-Barbosa D, et al. Bypassing the Greatwall-Endosulfine pathway: plasticity of a pivotal cell-cycle regulatory module in Drosophila melanogaster and Caenorhabditis elegans. Genetics 2012; 191:1181-97; PMID:22649080; http://dx.doi.org/10.1534/genetics.112.140574
- Li YH, Kang H, Xu YN, Heo YT, Cui XS, Kim NH, Oh JS. Greatwall Kinase Is Required for Meiotic Maturation in Porcine Oocytes. Biol Reprod 2013; 89(3):53.
- Adhikari D, Diril MK, Busayavalasa K, Risal S, Nakagawa S, Lindkvist R, Shen Y, Coppola V, Tessarollo L, Kudo NR, et al. Mastl is required for timely activation of APC/C in meiosis I and Cdk1 reactivation in meiosis II. J Cell Biol 2014; 206:843-53; PMID:25246615; http://dx.doi.org/10.1083/jcb.201406033
- Vigneron S, Gharbi-Ayachi A, Raymond AA, Burgess A, Labbe JC, Labesse G, Monsarrat B, Lorca T, Castro A. Characterization of the mechanisms controlling Greatwall activity. Mol Cell Biol 2011; 31:2262-75; PMID:21444715; http://dx.doi.org/10.1128/MCB.00753-10
- Blake-Hodek KA, Williams BC, Zhao Y, Castilho PV, Chen W, Mao Y, Yamamoto TM, Goldberg ML. Determinants for Activation of the Atypical AGC Kinase Greatwall during M Phase Entry. Mol Cell Biol 2012; 32:1337-53; PMID:22354989; http://dx.doi.org/10.1128/MCB.06525-11
- Yamamoto TM, Wang L, Fisher LA, Eckerdt FD, Peng A. Regulation of Greatwall kinase by protein stabilization and nuclear localization. Cell Cycle 2014; 13:3565-75; PMID:25483093; http://dx.doi.org/10.4161/15384101.2014.962942
- Wang P, Galan JA, Normandin K, Bonneil E, Hickson GR, Roux PP, Thibault P, Archambault V. Cell cycle regulation of Greatwall kinase nuclear localization facilitates mitotic progression. J Cell Biol 2013; 202:277-93; PMID:23857770; http://dx.doi.org/10.1083/jcb.201211141
- Wang P, Malumbres M, Archambault V. The Greatwall-PP2A axis in cell cycle control. Methods Mol Biol 2014; 1170:99-111; PMID:24906311; http://dx.doi.org/10.1007/978-1-4939-0888-2_6
- Santos SD, Wollman R, Meyer T, Ferrell JE, Jr. Spatial positive feedback at the onset of mitosis. Cell 2012; 149:1500-13; PMID:22726437; http://dx.doi.org/10.1016/j.cell.2012.05.028
- Pedruzzi I, Dubouloz F, Cameroni E, Wanke V, Roosen J, Winderickx J, De Virgilio C. TOR and PKA signaling pathways converge on the protein kinase Rim15 to control entry into G0. Mol Cell 2003; 12:1607-13; PMID:14690612; http://dx.doi.org/10.1016/S1097-2765(03)00485-4
- Wanke V, Pedruzzi I, Cameroni E, Dubouloz F, De Virgilio C. Regulation of G0 entry by the Pho80-Pho85 cyclin-CDK complex. EMBO J 2005; 24:4271-8; PMID:16308562; http://dx.doi.org/10.1038/sj.emboj.7600889
- Cundell MJ, Bastos RN, Zhang T, Holder J, Gruneberg U, Novak B, Barr FA. The BEG (PP2A-B55/ENSA/Greatwall) Pathway Ensures Cytokinesis follows Chromosome Separation. Mol Cell 2013; 52:393-405; PMID:24120663; http://dx.doi.org/10.1016/j.molcel.2013.09.005
- Vigneron S, Brioudes E, Burgess A, Labbe JC, Lorca T, Castro A. Greatwall maintains mitosis through regulation of PP2A. Embo J 2009; 28:2786-93; PMID:19680222; http://dx.doi.org/10.1038/emboj.2009.228
- Yamamoto TM, Blake-Hodek K, Williams BC, Lewellyn AL, Goldberg ML, Maller JL. Regulation of Greatwall kinase during Xenopus oocyte maturation. Mol Biol Cell 2011; 22:2157-64; PMID:21551066; http://dx.doi.org/10.1091/mbc.E11-01-0008
- Hegarat N, Vesely C, Vinod PK, Ocasio C, Peter N, Gannon J, Oliver AW, Novák B, Hochegger H. PP2A/B55 and Fcp1 regulate Greatwall and Ensa dephosphorylation during mitotic exit. PLoS Genet 2014; 10:e1004004; PMID:24391510; http://dx.doi.org/10.1371/journal.pgen.1004004
- Chen F, Archambault V, Kar A, Lio P, D'Avino PP, Sinka R, Lilley K, Laue ED, Deak P, Capalbo L, et al. Multiple protein phosphatases are required for mitosis in Drosophila. Curr Biol 2007; 17:293-303; PMID:17306545; http://dx.doi.org/10.1016/j.cub.2007.01.068
- Heim A, Konietzny A, Mayer TU. Protein phosphatase 1 is essential for Greatwall inactivation at mitotic exit. EMBO Rep 2015; 16:1501-10; PMID:26396231; http://dx.doi.org/10.15252/embr.201540876
- Archambault V, D'Avino PP, Deery MJ, Lilley KS, Glover DM. Sequestration of Polo kinase to microtubules by phosphopriming-independent binding to Map205 is relieved by phosphorylation at a CDK site in mitosis. Genes Dev 2008; 22:2707-20; PMID:18832073; http://dx.doi.org/10.1101/gad.486808
- Daul AL, Komori H, Lee CY. Immunofluorescent staining of Drosophila larval brain tissue. Cold Spring Harb Protoc 2010; 2010:pdb prot5460
- Nissim-Rafinia M, Meshorer E. Photobleaching assays (FRAP & FLIP) to measure chromatin protein dynamics in living embryonic stem cells. J Vis Exp 2011; PMID:21730953
- D'Avino PP, Archambault V, Przewloka MR, Zhang W, Laue ED, Glover DM. Isolation of protein complexes involved in mitosis and cytokinesis from Drosophila cultured cells. Methods Mol Biol 2009; 545:99-112; PMID:19475384; http://dx.doi.org/10.1007/978-1-60327-993-2_6
- Fu SC, Huang HC, Horton P, Juan HF. ValidNESs: a database of validated leucine-rich nuclear export signals. Nucleic Acids Res 2013; 41:D338-43; PMID:23093589; http://dx.doi.org/10.1093/nar/gks936
