Nucleosomes are substrates for cellular signaling. This allows efficient coordination among different cells in response to signaling, which is received and delivered by chromatin modifying enzymes. Importantly, the catalytic activities of a majority of these enzymes, such as histone methyltransferases and acetyltransferases require cofactors, which are generated in the process of amino acid metabolism.
A chromatin modifying complex that alters nucleosomes and also generates cofactors for other chromatin modifying complexes has been discovered. SESAME (Serine-responsive SAM-containing metabolic enzyme complex) was purified from S. cerevisiae and found to contain pyruvate kinase (Pyk1), serine metabolic enzymes (Ser33, Ser3, and Shm2), SAM (S-adenosylmethionine) synthetases (Sam1 and Sam2) and an acetyl-CoA synthetase (Acs2).Citation1 SESAME connects chromatin modification and transcription regulation with cellular metabolism. Pyk1, a homologue of human PKM2, converts phosphoenolpyruvate (PEP) and ADP to pyruvate and ATP in glycolysis. However, Pyk1 in SESAME also uses PEP to phosphorylate histone H3 tyrosine 11 (H3T11). Both activities are stimulated by the presence of serine.Citation1 SESAME associates with the Set1 H3K4 methyltransferase complex, which requires SAM synthesized from SESAME. The requirement of SAM from SESAME was unique to Set1 mediated H3K4 methylation (H3K4me), which is stimulated by serine via SESAME. The Set1 complex recruits SESAME to target genes including the PYK1 gene, resulting in phosphorylation of H3T11 (H3pT11). H3pT11 plays several roles on the PYK1 gene. H3pT11 on the promoter, which highly responds to supplying serine or glucose in the culture media, is required for recruitment of Set1 complex and the activation of PYK1 transcription. However, H3pT11 on 5' gene body, where SESAME directly bound to nucleosomes, suppresses transcription, thus functions as a negative feedback for Pyk1 levels. Thus, SESAME regulates crosstalk between H3K4me and H3pT11 by sensing glycolysis and glucose-derived serine metabolism.
Different pyruvate kinase isoforms have different roles. In yeast, glucose induces Pyk1 expression and suppresses Pyk2.Citation1 Replacing PKM2 with PKM1, a yeast Pyk2 homologue, reversed the elevated aerobic glycolysis in tumor cells, indicating PKM1 and PKM2 play different roles in glycolysis.Citation1 Using non-immortalized primary cells from PKM2 conditional mice Lunt, S. et al. found that PKM1 unique expression via deleting PKM2 leads to cell proliferation arrest.Citation2 Interestingly, the unique expression of PKM1 suppresses nucleotide biosynthesis, and supplying exogenous thymine (5-methyl uracil) restores the proliferation of arrested embryonic fibroblasts from these mice.Citation2 A function of PKM2 in nucleotide biosynthesis has also been discovered. Keller, K. et al. found that PKM2 directly interacts with succinyl-5-aminoimidazole-4-carboxamide-1-ribose-5'-phosphate (SAICAR), an intermediate of the de novo purine nucleotide biosynthesis. Binding of SAICAR stimulates PKM2 kinase activity.Citation3 The serine/glycine hydroxymethyltransferase (SHM), a homologue of the Shm2 SESAME subunit, functions in the thymine nucleotide synthesis where it converts serine to glycine. Thus, it will be interesting to determine if SESAME plays a role in nucleotide biosynthesis. Curiously, both SAICAR-PKM2 and SESAME carry both pyruvate kinase activity and H3T11 kinase activity. The levels of H3pT11 correlate with cellular levels of SAICAR, suggesting purine nucleotide synthesis is reflected in the kinase activity of PKM2.Citation3 It is still unknown whether nucleotide biosynthesis is consequence of pyruvate synthesis as an event of cellular metabolism, or whether nucleotide biosynthesis is directly sensitive to PEP, which donates phosphate to PKM2 and also other kinases that may regulate nucleotide biosynthesis. The question of whether transcription regulation is connected to nucleotide biosynthesis will need to be addressed.
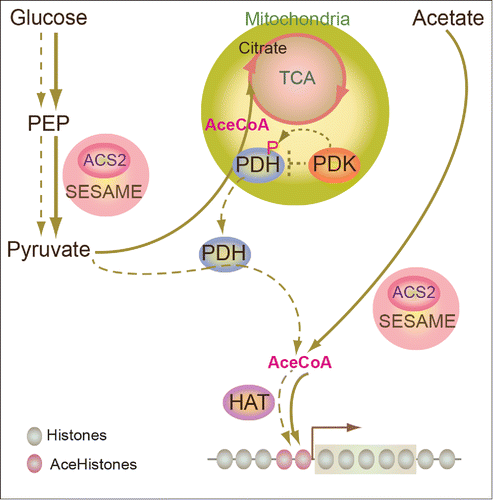
The mechanism by which the sensing of pyruvate synthesis regulates cellular energy consumption has been studied. Pyruvate dehydrogenase (PDH, E1) in mitochondria exists in a complex (PDC) with dihydrolipoamide (E2), dihydrolipoamide dehydrogenase (E3) and E3-binding protein (E3BP). PDH regulates pyruvate entering the TCA cycle. The inhibition of PDH via its phosphorylation by pyruvate dehydrogenase kinase (PDK) reduces the amount of pyruvate entering the TCA cycle, causing a reduction of mitochondrial oxidation of pyruvate and reducing oxidativephosphorylation.Citation4–6 PDC has also been found to play a key role for nuclear histone acetylation.Citation5 PDC can be translocated from mitochondria to the nucleus, and this translocation is promoted by mitochondrial stress.Citation5 Nuclear PDC contributes to the import of the de novo acetyl-CoA synthase and nuclear histone acetylation during G1-S phase progression.Citation5 Acetyl-CoA is synthesized by Acetyl-CoA synthetase (AceCS2 in human and ACS in yeast) and by ATP-citrate lyase.Citation7 The levels of acetyl-CoA represent metabolic states.Citation7 SESAME contains ACS2, however the roles of ACS2 in the SESAME are still unknown. A functional connection between PDC and SESAME can be predicted. If PDC functionally coordinated with SESAME in response to metabolic states, the linkage of these machineries may explain how chromatin modification and transcription regulation are influenced with the energy (ATP) generation promoted by cellular metabolism (). In other words, chromatin modifications may reflect the cellular metabolic status to trace the energy necessary for transcription regulation.
Figure 1. Hypothetical functions of SESAME in yeast acetyl-CoA synthesis. Through supplying glucose, pyruvate generated by SESAME is utilized by PDH to synthesize acetyl-CoA, which feeds the TCA cycle. During glucose starvation, PDH is translocated from mitochondria to the nucleus and provides acetyl-CoA for histone acetylation. Solid arrow indicates the condition supplied with glucose. Dashed arrow indicates the condition during glucose starvation.
In tumorigenesis, suppression of elevated expression of phosphoglycerate dehydrogenase, a mammalian homologue of the Ser33 SESAME subunit, decreased cell proliferation. Moreover, reduction of PKM2 expression has been targeted for tumor therapy.Citation1 Importantly, SESAME functions as a feedback loop for Pyk1 expression. Thus, examination of SESAME activities, such as measuring SAM and pyruvate and defining mutations in its components may prove useful for the prediction and therapy of tumors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Li S, et al. Mol Cell 2015; 60:408–21; PMID:26527276; http://dx.doi.org/10.1016/j.molcel.2015.09.024
- Lunt SY, et al. Mol Cell 2015; 57:95–107; PMID: 25482511; http://dx.doi.org/10.1016/j.molcel.2014.10.027
- Keller KE, et al. Mol Cell 2014; 53:700–9; PMID:24606918; http://dx.doi.org/10.1016/j.molcel.2014.02.015
- Behal RH, Buxton DB, Robertson JG, Olson MS. Regulation of the pyruvate dehydrogenase multienzyme complex. Annu Rev Nutr 1993; 13:497–520; PMID:8369156; http://dx.doi.org/10.1146/annurev.nu.13.070193.002433
- Sutendra G, et al. Cell 2014; 158:84–97; PMID:24995980; http://dx.doi.org/10.1016/j.cell.2014.04.046
- Holness MJ, et al. Biochemical Society transactions 2003; 31:1143–51; PMID:14641014; http://dx.doi.org/10.1042/bst0311143
- Comerford SA, et al. Acetate dependence of tumors. Cell 2014; 159:1591–602; PMID:25525877; http://dx.doi.org/10.1016/j.cell.2014.11.020
