Integrins are highly abundant cell surface adhesion receptors expressed in all cell types but erythrocytes in mammals. They are heterodimeric molecules formed of a single transmembrane α- and β-subunit and mediate cell interactions with extracellular matrix (ECM) molecules. Integrins regulate many physiological events, such as cell motility, cell survival, proliferation and gene expression.Citation1 Integrins are unique from other receptors in their ability to undergo bidirectional signaling. Intracellular signals can be transmitted to the receptors to initiate the conformational activation. This processes, referred to as inside-out signaling, is mediated by cytoplasmic proteins like talin binding to the integrin β-cytoplasmic tail. The activated receptor can engage ECM ligands and initiate so called outside-in signaling which involves activation of downstream signaling pathways and coupling of the receptors to the actin cytoskeleton. Ligand-bound integrins cluster to form large signaling and mechanotransduction protein complexes called focal adhesions (FA). Integrin signaling in response to ECM adhesion activates focal adhesion kinase (FAK) as well as the ERK-MAPK and PI3K-AKT pathways. These signals regulate many cellular processes, including anchorage-dependent cell survival.
We and others Citation2,3 have shown that integrins undergo constant endosomal traffic. Both active and inactive integrins are endocytosed from the plasma membrane and recycled back to facilitate cell migration, invasion and cytokinesis. Active integrins bound to ligand fragments have been detected in endosomal compartments.Citation2 However, the prevailing idea has been that integrin signaling would be restricted to the plasma membrane FAs. Our recent findings expand the concept of integrin signaling from the plasma membrane to the endosomes.Citation4 We find that similarly to many growth-factor activated receptor tyrosine kinases (RTKs), integrin signaling is coupled to endocytosis. Inhibition of integrin internalization via different means, including dynamin inhibition and Rab21 silencing, strongly attenuates adhesion induced signals in normal human fibroblasts as well as human cancer cell lines.Citation4 In line with this, EEA1- positive endosomes contain active integrin, ECM ligands and focal adhesion kinase. Thus, integrin downstream signaling is not restricted to the plasma membrane. Interestingly, the endosomal integrin signaling components are distinct from the core focal adhesion components. Talin, a critical regulator of integrin activity, localizes to the endosomes but paxillin, vinculin as well as active Src kinase are not enriched on endosomes Citation4 ().
Figure 1. Integrins signal from endosomes. Rab21-mediated integrin endocytosis to EEA1- endosomes is required for full ECM/integrin-induced signaling. The FAK FERM-domain, but not the focal adhesion targeting (FAT) domain, is targeted to endosomes alone and thus endosomal recruitment of FAK is likely to occur via the FERM. Endosomal FAK signaling suppresses anoikis and supports anchorage-independent growth and metastasis of breast cancer cells.
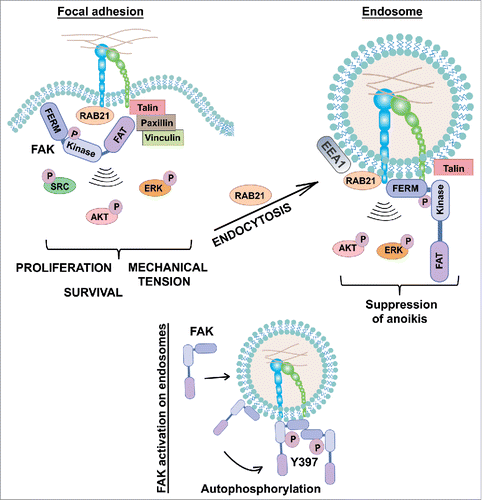
Normal adherent cells are unable to survive upon loss of integrin-mediated adhesion and undergo anoikis, a specific form of programmed cell death.Citation1 Resistance to anoikis is among the many hallmarks of cancer and is essential for cancer dissemination. Upon cell detachment, integrin containing adhesions and signaling on the plasma membrane are lost. However, the signaling of endocytosed integrins persists longer and functions to delay the onset of anoikis in normal cells.Citation4 Correspondingly, integrin endosomal activation of FAK facilitates anchorage-independent growth of cancer cells in vitro and is essential for their ability to metastasise from veins in mice, presumably by supporting their survival in circulation. There are several reports of constitutively activated FAK in cancer cells.Citation5 It is likely that such adhesion-independent persistent FAK activity could be linked to endosomal signaling. This would be in line with the notion that cancer cells display increased endocytosis and enlarged endosomal compartments, implicated already in endosomal signaling of activated RTKs for example.Citation6
While is it now evident that integrin traffic is not only a pathway for receptor transport but also a critical contributor to their signaling,Citation4 many of the mechanistic details remain unknown. Using in vitro reconstitution experiments we found that FAK can be recruited directly to endosomes, where it undergoes autophosphorylation and activation. Furthermore, early endosomal antigen-1 (EEA1) was required for the integrin endosomal signaling to FAK. However, the exact mechanism of endosomal FAK targeting remains to be studied. Interestingly, FAK targeting to the endosomes is dependent on the N-terminal FERM-domain of FAK, which contains the integrin binding site.Citation4 This is distinct from FA targeting which is mediated by the C-terminal FAT-domain of FAK. Thus, it is possible that integrin endosomal signaling is not merely a continuum of plasma membrane initiated events but rather the outcome of distinct endosomal integrin signaling complex assembly.
Several proteomic studies have contributed to defining the core components of the FAs i.e. the adhesome.Citation7 Proteomic studies of the integrin-positive endosome-containing membrane fractions Citation4 revealed that many integrin and FAK binding proteins are enriched on endosomes. Future studies will be needed to define which of these are the core components of the “endoadhesomes.” Another open and exciting avenue for investigation will be defining the exact signals emanating from the endosomal integrins and determining their biological function – other than suppression of anoikis.
References
- Harburger DS, Calderwood DA. J Cell Sci 2009; 122:159-63; PMID:19118207; http://dx.doi.org/10.1242/jcs.018093
- De Franceschi N, et al. J Cell Sci 2015; 128:839-52; PMID:25663697; http://dx.doi.org/10.1242/jcs.161653
- Paul NR, et al. Curr Biol 2015; 25:R1092-105; PMID:26583903; http://dx.doi.org/S0960-9822(15)01160-4[pii]
- Alanko J, et al. Nat Cell Biol 2015; 17:1412-21; PMID:26436690; http://dx.doi.org/10.1038/ncb3250
- Sulzmaier FJ, et al. Nat Rev Cancer 2014; 14:598-610; PMID:25098269; http://dx.doi.org/10.1038/nrc3792
- Scita G, Di Fiore PP. Nature 2010; 463:464-73; PMID:20110990; http://dx.doi.org/10.1038/nature08910
- Horton ER, et al. Nat Cell Biol 2015; 17(12):1577-87; PMID:26479319; http://dx.doi.org/10.1038/ncb3257
