Embryonic stem cells (ESCs) can differentiate into all cell types of the body (pluripotency), and self-renew long term, if not infinitely, under adequate conditions in vitro. Their self-renewal and differentiation is controlled by culture conditions, and their differentiation seems to resemble that of cells in the embryo well. ESCs are therefore an excellent experimental in vitro model of both, stem cell control, and embryonic development.Citation1 They also hold huge potential in disease modeling and regenerative medicine. Their fast proliferation and easy genetic manipulation make them highly accessible for high throughput approaches in biochemistry, molecular biology, biophysics and cell biology. ESCs are therefore a favorite mammalian stem cell model of systems biology to study the molecular control of cell states and their transitions, and the design of molecular networks.Citation2
Self-renewal of the pluripotent state is governed by a circuitry of transcription factors (TFs) that is controlled by external signals.Citation1 The pluripotency gene regulatory network (PGRN) is complex and rich in feed-back and feed-forward loops.Citation2 Expression of Nanog, a core TF of the PGRN can be high or low in ESCs. It is thought that this correlates with low and high ESC differentiation probabilities, respectively (Chambers, 2007). This led to the hypothesis that heterogeneity of ESC populations is functionally important to maintain pristine self-renewing cells and at the same time generate cells poised for differentiation. Importantly, cell fate decision making is inherently a dynamic process of single cells - not of static population averages. Thus, to determine the control of cell behavior, individual cells must be continuously measured.Citation3 We therefore quantified Nanog protein expression dynamics in individual mouse ESCs over many generations.Citation4
Transitions from negative to high Nanog expression typically take longer than one cell generation, do not happen as part of oscillations and occur independently of the cell cycle phase. Nanog fluctuations are very heterogeneous between individual cells. Transitions between Nanog expression states happen with certain probabilities that, at the population level, remain constant in self-renewing ESCs. However, when analyzing Nanog expression dynamics in single cells, they can behave very differently from what would be predicted from population averages. In particular, we identified a subpopulation of Nanognegative ESCs that remains Nanog negative or low for many generations. Importantly, while Klf4 is often absent in these cells, Oct4 and Sox2 are still expressed at lower, but considerable levels. Interestingly, and in contrast to other ESC populations, Oct4 and Klf4 are negatively correlated in those cells. They also have different differentiation properties than other ESCs, with reduced endodermal or neurectodermal differentiation. Importantly, Nanognegative cells in colonies in which some cells express Nanog are different from Nanognegative cells in the colonies without any Nanog upregulation over many generations (). Thus, 2 functionally distinct subpopulations of Nanognegative cells exist: one, which fluctuates between negative/low and high Nanog expression states without changing its differentiation propensity; and one, which does not revert to the Nanoghigh state and responds to differentiation cues differently. Those findings challenge the prevailing view that the Nanognegative state represents a “window of opportunity” which is poised to differentiation. This paradigm was mainly based on the finding that Nanognegative cells produce less undifferentiated colonies.Citation5 Our data suggest that this “metastable” Nanognegative state could be an artifact of the population average of Nanognegative cells. We hypothesize that ESCs fluctuating between different Nanog expression states are functionally more similar than previously thought, and that previously suggested functional differences are at least in part due to the non-fluctuating Nanognegative cells. Our data suggest caution when inferring fluctuations of separately and statically measured PGRN factors or epigenetic states from measured fluctuations of individual pluripotency factors like Nanog. It is necessary to measure the dynamics of these factors, or at least link them directly to other cell features that were measured in the same individual cells over time. Nanog dynamics – in contrast to static Nanog levels – can distinguish some ESC states like destructive high-dimensional gene expression analyses.
Figure 1. ESCs can transition between different Nanog expression states. Within the Nanognegative/low ESC population, different states exist: cells that do upregulate Nanog again, and those that do not for many generations. These different Nanognegative/low states differ in their differentiation propensities. Nanog upregulation is not well correlated to Oct4, Sox2 and Klf4 expression, or cell cycle phase.
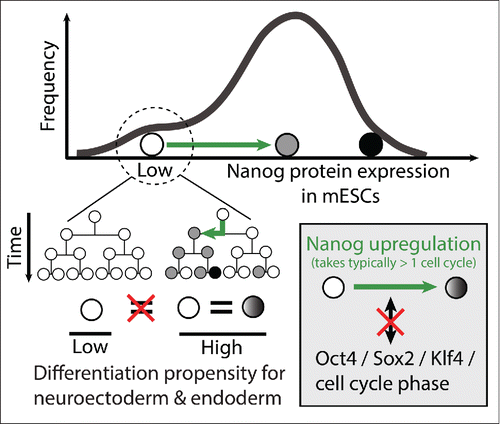
Nanog upregulation does not correlate with the expression levels of the PGRN TFs Oct4, Sox2, Klf4, or cell cycle phase.Citation4 Manipulations of Nanog levels showed its importance as a pluripotency factor,Citation6 but a tight deterministic regulation within the PGRN – at least in self-renewal conditions – and its use as a cell fate predictor are questionable. In contrast, our data agree with the notion that pluripotency is represented by an attractor basin in which ESCs are moving while cells that stay long-term low for Nanog are in a different attractor state with reduced developmental potential.Citation7
It will be desirable to increase the number of simultaneously measured pluripotency and differentiation regulators and to find reliable dynamic expression patterns that correlate well with large-scale expression changes. Additionally, precise experimental control of external signals and expression levels of PGRN TFs will be necessary to better define the causative relationships within the PGRN that eventually control single-cell decisions makings.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Martello G, Smith A. Annu Rev Cell Dev Biol 2014; 30:647-75; PMID:25288119; http://dx.doi.org/10.1146/annurev-cellbio-100913-013116
- Macarthur BD, et al. Nat Rev Mol Cell Biol 2009; 10:672-81; PMID:19738627; http://dx.doi.org/10.1038/nrm2766
- Etzrodt M, et al. Cell Stem Cell 2014; 15:546-58; PMID:25517464; http://dx.doi.org/10.1016/j.stem.2014.10.015
- Filipczyk A, et al. Nat Cell Biol 2015; 17:1235-46; PMID:26389663; http://dx.doi.org/10.1038/ncb3237
- Chambers I, et al. Nature 2007 [cited 2014 Jul 12]; 450:1230-4; PMID:18097409; http://dx.doi.org/10.1038/nature06403
- Mitsui K, et al. Cell 2003; 113:631-42; PMID:12787504; http://dx.doi.org/10.1016/S0092-8674(03)00393-3
- Semrau S, van Oudenaarden A. Annu Rev Cell Dev Biol 2015; 31:317-45; PMID:26566114; http://dx.doi.org/10.1146/annurev-cellbio-100814-125300
