Since it was first described as a protein module in pleckstrin over 20 y ago, the pleckstrin homology (PH) domain has been found in about 285 proteins and its involvement in diverse physiological processes has been shown.Citation1,2 This 100-residue module is structured as a seven-strand antiparallel β-sheet and C-terminal α-helix.Citation3
The PH domain specifically binds phosphatidylinositol-4,5-bisphosphate,Citation4 indicating its ability to transiently anchor proteins to intracellular membrane surfaces and participate in recruiting proteins present in cell cytoplasm to the surface of organelles. Moreover, some PH domains associate directly with G-protein coupled receptors (GPCR), allow Dbl homology (DH) domains to regulate Rho-family GTPase exchange factors (GEFs), or bound to the C-terminal site of the focal adhesion kinase (FAK) FERM domain. The role of a PH-like super-fold as a universal module mediating both protein-protein and protein-membrane associations is supported by the finding that most yeast PH domains do not bind to phospholipids and do not recruit proteins to membranes.
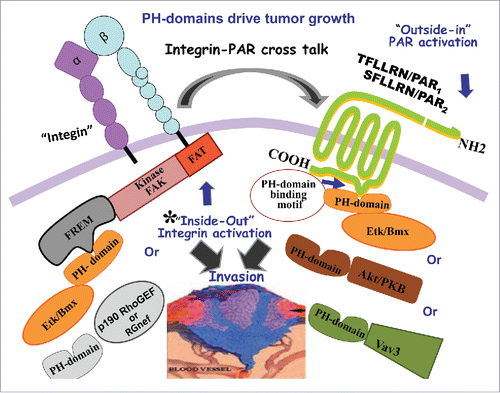
We have recently shown the direct association of PH-domains with members of a GPCR subfamily known as protease-activated receptors, or PARs, which are critical in modulating PAR signaling in cancer growth.Citation5 This occurs via the binding of Akt/PKB-PH domain as a key-signaling event of PARs. Other PH-domain signal proteins such as Etk/Bmx and Vav3 also associate with PAR1 and PAR2 through their PH domains (). In PAR2, a H349A, but not a R352A point mutation, abrogates PH-protein association and is sufficient to markedly reduce PAR2-instigated breast tumor growth in vivo and placental extravillous trophoblast (EVT) invasion in vitro. Similarly, the PAR1 mutant hPar1-7A, which is unable to bind PH-domain, markedly inhibits mammary tumor development and EVT invasion, endowing these motifs with physiological significance and underscoring the importance of these previously unknown PAR1 and PAR2 PH-domain binding motifs in both pathological and physiological invasion processes.
Figure 1. PH-domains drive tumor growth. Activation of PARs (1and2) lead to PH-signal protein association. These binding motifs are critical for tumor growth. Inside-out activation of integrins via PH-Etk/Bmx-FERM/FAK or PH-Rgnef-FAK can initiate cross- talk with PARs, during cancer growth.
A lot is known about the interactions mediated by DH-PH scaffolds providing opportunities to regulate GTPase activity during cell signaling throughout the tandem DH-PH module, which often binds the GDP-bound RhoA (inactive status) and stabilizes the nucleotide-free form until GTP (active status) is released. p63RhoGEF, for example, is directly activated by members of the Gαq subfamily of G proteins and is thus regulated by GPCRs. The PH-domain modules effectively associate with GPCRs and tyrosine kinase receptors (TKR), as well as with a wide spectrum of cell signal proteins.
Another basic aspect of cell surface receptor functions involves the biology of adhesion to the extracellular matrix (ECM) basement membrane. The integrin family of transmembrane receptors links the ECM to the intracellular actin cytoskeleton at focal adhesion interaction points.Citation6 Among the most studied integrin-activated protein tyrosine kinase (PTK) is focal adhesion kinase (FAK). FAK is composed of the N-terminal FERM (band 4.1, ezrin, radixin, moesin homology) domain, a central PTK region, proline-rich regions, and a C-terminal domain that links FAK to integrins. The PH-domain found within Etk/Bmx signal protein binds directly to FAK-FERM domain mediated via direct protein-protein interactions.
Another FAK PH domain protein-protein interaction was shown to occur using the Rgnef (also known as p190RhoGEF) (17). It was shown that Rgnef binds specifically FAK through its PH-domain. Rgnef-FAK interaction facilitates cell adhesion, and a mutation in the Rgnef PH-domain inhibits adhesion.
Interestingly, the PH-domain association with PARs Citation5 is both lipid-dependent, as is the case of Akt-PH association, and lipid-independent mediated via protein-protein association, as shown for PH-Etk/Bmx. One possible explanation is that once PAR C-tail recruits the PH-domain signal protein, targeting to the membrane is mediated via palmitoylation of a cysteine residue in the PAR1and2C-tail. The highly conserved eighth helix (H8) previously identified in rhodopsin and other Class A receptors, including PAR1 and PAR2, is located in the PAR C-terminal domain. In fact, the PAR1 PH-domain binding site is localized within the H8 loop and is confined to this region. Similarly, palmitoylation of PAR2 is necessary for post-translational modification and is required for efficient cell surface expression and desensitization of PAR2.
In summary, PH motifs that provide pivotal binding modules, either with lipids or directly via protein-protein interactions, exemplify how the interplay between distinct motifs in a signal protein not only support transmission of a biochemical signal, but also ensure a robust response to developmental cues, at precisely the right time and with sufficient specificity to safeguard against premature and hence disastrous induction of cell fate change. Once activated, cell surface receptors playing a central role in tumor biology, such as the PARs, recruit PH-domain signal proteins to relay appropriate cell signaling. PAR may also crosstalk with integrins.Citation7 The complex formation of FAK-PH-Etk/Bmx, as well as FAK-PH-Rgnef, may initiate inside-out signaling that affects the extracellular portion of the integrin and “activates” it. Activated integrins can cross associate with PARs (), which subsequently transmit signaling in an outside-in manner and associate with PH-signal protein/s.
References
- Mayer BJ, et al. Cell 1993 May 21; 73(4):629–30; PMID:8500161; http://dx.doi.org/10.1016/0092-8674(93)90244-K
- Haslam RJ, et al. Nature 1993 May 27; 363(6427):309–10; PMID: 8497315; http://dx.doi.org/10.1038/363309b0
- Yoon HS, et al. Nature 1994 Jun 23; 369(6482):672–5; PMID:8208296; http://dx.doi.org/10.1038/369672a0
- Harlan JE, et al. Nature 1994 Sep 8; 371(6493):168–70; PMID: 8072546; http://dx.doi.org/10.1038/371168a0
- Kancharla A, et al. Nat Commun 2015 Nov 24; 6:8853; PMID: 26600192; http://dx.doi.org/10.1038/ncomms9853
- Geiger B, Yamada KM. Cold Spring Harb Perspect Biol 2011 May 1; 3(5):a005033; PMID:21441590; http://dx.doi.org/10.1101/cshperspect.a005033
- Even-Ram SC, et al. J Biol Chem 2001 Apr 6; 276(14):10952–62; PMID:11278329; http://dx.doi.org/10.1074/jbc.M007027200
