It was 1906 when Dr. Alois Alzheimer', a German psychiatrist and neuropathologist, analyzed the brain of his late patient, Auguste Deter who suffered from loss of short term memory and abnormal behavior. He observed histological changes in the brain that were later defined as amyloid plaques and neurofibrillary tangles, two features that set the basis for the definition of a new disease which bears his last name, “Alzheimer's disease” (AD). Nowadays AD is the most prevalent cause of dementia in the industrialized world. Although a wealth of information regarding the disease and its causes has been accumulated over the years, key mechanistic questions remain only partially answered. What molecular mechanisms underlie the development of this devastating disorder and whether one mechanism is accountable for all cases or rather, different traits lead to the same unfortunate fate, are among these mysteries.
A major breakthrough in AD research was achieved when a family of aggregative peptides, known as Amyloid Beta (Aβ) peptides, were identified as the major component of the amyloid plaques that are typically seen in brains of AD patients.Citation1 Later it was discovered that Aβ peptides are proteolytic products of a trans-membranal protein which was termed the Amyloid Precursor Protein (APP).Citation2 Two proteases, the β Amyloid Cleaving Enzyme (BACE) and the γ secretase proteolytic complex digest APP to release the various forms of Aβ peptides. While the vast majority of AD cases onset sporadically, numerous mutations in APP, presenilin 1 or presenilin 2 (both presenilins are components of the γ secretase complex) underlie the development of early onset, familial AD (fAD). Despite their rarity, mutation-linked AD cases provide invaluable hints that can help decipher the mechanisms underlying the development of this degenerative disorder.
The understanding that Aβ-containing plaques are correlated with AD has led to the development of the “amyloid hypothesis,” a theory which proposes that fAD-linked mutations in PS1 or PS2 modulate Aβ metabolism, oligomerization and deposition in plaques. The accumulation of Aβ was proposed to impair neuronal activity, cause cell loss and eventually underlie the manifestation of AD.Citation3 Although the amyloid hypothesis is supported by some evidence, recent studies challenge this theory, suggesting that at least some fAD cases emanate from mutations in the sequence of presenilin 1 which lead to loss of γ secretase activity. First, it was shown that mice that harbor two copies of either the L435F or C410Y fAD-linked mutated presenilin 1 exhibit near complete loss of γ secretase function but develop neurodegeneration.Citation4 In addition, the analysis of γ secretase activity in brains of individuals who suffered from fAD unveiled that in most cases the total production of Aβ was lower than the levels seen in brains of individuals who showed no signs of dementia (control brains). Surprisingly, no difference in total Aβ levels was seen among brains of individuals who had sporadic AD and control brains.Citation5 These studies raise the questions of how fAD-inducing mutations lead to the loss of γ secretase activity and what are the consequences of such loss at the cellular level.
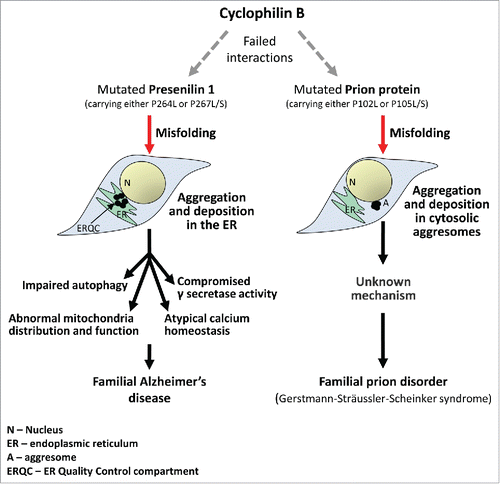
Since attaining the correct folding is critical for protein maturation and functionality we asked whether presenilin 1 misfolding may play a role in the development of AD. To identify mechanisms that impair presenilin 1 folding, we compared neurodegeneration-causing mutations in different proteins. This approach is based on the rationale that since folding chaperones serve many client polypeptides, it is plausible that an aging-associated decline in their activity impairs the folding of different neurodegeneration-causing proteins. Thus, mutations in chaperone recognition sites prevent the client polypeptides from attaining their correct spatial structures, leading to misfolding, aggregation and disease. We found similar PXXP motifs in the sequences of the prion protein (PrP) and of presenilin 1. The substitution of either proline in these motifs of PrP and presenilin 1 underlies the development of familial prion disorder or Alzheimer's disease respectively.Citation6 Visualizing cells that express mutated presenilin 1 molecules carrying the fAD-linked proline substitutions (P264L or P267L/S) we found that the mutated proteins form aggregates that are deposited in the endoplasmic reticulum. Cells that were derived from a presenilin knockout mouse and in which the mutated protein was expressed, exhibited significantly reduced γ secretase activity and impaired mitochondrial distribution and function.Citation6 Since presenilin 1 is known to be involved in the promotion of additional key biological activities,Citation7 it is likely that the aggregation of this protein impairs different aspects of cellular function and homeostasis.
Our study supports the notion that loss of γ secretase activity underlies certain fAD cases and illuminates a few important aspects in the biology of neurodegenerative disorders (). First, we show that one mechanistic event, a failed interaction of a client protein with cyclophilin B, underlies the development of two distinct familial neurodegenerative maladies. In addition, this mechanism is unrelated to the manifestation of AD in individuals who carry other fAD-causing mutations, indicating that more than one cellular mechanism is accountable for the development of this devastating disease.
Figure 1. The chaperone, cyclophilin B is critical for the proper maturation of the prion protein (PrP) and of presenilin 1. The substitution of proline 102 or 105 leads to PrP misfolding, aggregation and deposition in cytosolic aggresomes, resulting in the development of the Gerstmann-Sträussler-Scheinker syndrome. Similarly, the substitution of proline in residues 264 or 267 of presenilin 1 prevents cyclophilin B from assisting its folding, causing aggregation, deposition in the ER and loss of γ secretase function. This impairs mitochondrial distribution and activity, as well as other cellular functions and underlies the manifestation of certain cases of familial Alzheimer's disease.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Masters CL, et al. Proc Natl Acad Sci U S A 1985; 82:4245-9; PMID:3159021; http://dx.doi.org/10.1073/pnas.82.12.4245
- Kang J, et al. Nature 1987; 325:733-6; PMID:2881207; http://dx.doi.org/10.1038/325733a0
- Hardy JA, Higgins GA. Science New York 1992; 256:184-5; http://dx.doi.org/10.1126/science.1566067
- Xia D, et al. Neuron 2015; 85:967-81; PMID:25741723; http://dx.doi.org/10.1016/j.neuron.2015.02.010
- Szaruga M, et al. J Exp Med 2015; 212:2003-13; PMID:26481686; http://dx.doi.org/10.1084/jem.20150892
- Ben-Gedalya T, et al. EMBO J 2015; 34:2820-39; PMID:26438723; http://dx.doi.org/10.15252/embj.201592042
- De Strooper B, et al. Annu Rev Cell Dev Biol 2010; 26:235-60; PMID:20604710; http://dx.doi.org/10.1146/annurev-cellbio-100109-104117
