ABSTRACT
The biological behaviors of hepatocellular carcinoma (HCC) are complex mainly due to heterogeneity of progressive genetic and epigenetic mutations as well as tumor environment. Hepatocyte growth factor (HGF)/c-Met signaling pathway is regarded to be a prototypical example for stromal-epithelial interactions during developmental morphogenesis, wound healing, organ regeneration and cancer progression. And p53 plays as an important regulator of Met-dependent cell motility and invasion. Present study showed that 2 HCC cell lines, Hep3B and HepG2, displayed different invasive capacity when treated with HGF which was secreted by hepatic stellate cells (HSCs). We found that HGF promoted Hep3B cells invasion and migration as well as epithelial-mesenchymal transition (EMT) occurrence because Hep3B was p53 deficient, which leaded to the c-Met over-expression. Then we found that HGF/c-Met promoted Hep3B cells invasion and migration by upregulating Snail expression. In conclusion, HGF/c-Met signaling is enhanced by loss of p53 expression, resulting in increased ability of invasion and migration by upregulating the expression of Snail.
Introduction
Despite of the treatments of hepatocellular carcinoma (HCC) have obtained constantly advancement in recent years, such as improvement of surgical resection, application of chemotherapy, radiofrequency ablation, liver transplantation and so on, the outcome of HCC remains in gray.Citation1,2 The biological behaviors of HCC are complex mainly due to heterogeneity of progressive genetic and epigenetic mutations as well as tumor environment which the present researches have focused on.Citation3,4 The present perspective considers that the hepatocarcinogenesis and development is a multistep, ongoing process and a series of oncogenes and tumor suppressor genes alterations are agitated. Meanwhile, factors derived from tumor stromal assist in facilitating HCC progression by forming an extraordinary environment to support tumor growth, invasion and migration. Therefore, understanding the basic principles of tumor biology and its interactions with the microenvironment increase insights into the mechanism of tumor invasion and migration.
The liver tumor microenvironment is a complex mixture of tumoral cells within the extracellular matrix (ECM), combined with stromal cells and the cytokines they secrete. Prior studies have identified the importance of hepatic stellate cells (HSCs) in HCC progression, including tumorigenesis, growth, invasion and migration.Citation5-7 HSCs are provided with migratory ability to infiltrate the tumor stromal and release growth factors including hepatocyte growth factor (HGF).Citation8 HGF is a multifunctional cytokine that expresses ubiquitously and binds to the receptor tyrosine kinase Met to impact cell proliferation, scattering, survival and migration.Citation9-11 In mammal animals, HGF/c-Met signaling is essential for normal embryonic development and adult tissue repair and remained at a certain level in normal tissue.Citation12,13 However, inappropriate activation of HGF/c-Met signaling, amplification of the c-Met gene, with consequent protein overexpression and constitutive kinase activation which may lead to tumorigenesis, growth, invasion and migration.Citation14
The p53, a sequence-specific DNA binding transcription factor is a multifunctional tumor suppressor which triggers several biologic responses including cell cycle arrest, apoptosis, senescence and differentiation to response to cellular stress such as DNA damage, oncogene activation, hypoxia and telomerase erosion.Citation15 In human body, several hundred genes are regulated by p53, including c-Met.Citation16-18 There have been reported that MET-dependent cell motility and invasion are controlled by tumor suppressor p53.Citation15
In this article, we found that HCC cell line Hep3B was more sensitive to HGF which was secreted by HSCs than HepG2. This feature was decided by the different expression of c-Met and p53. Met overexpression was regulated by p53 deficiency. And we found that HGF/c-Met signaling promoted Hep3B cells invasion and migration through Snail activation.
Results
LX-2 cells promoted invasion and migration of Hep3B cells, but not HepG2 cells
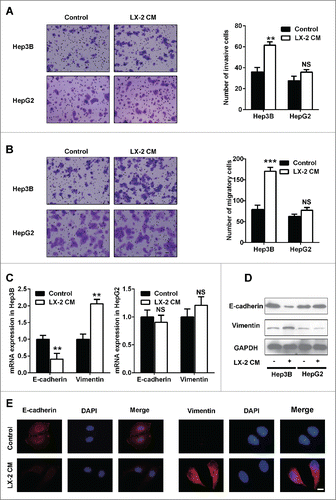
To investigate the role of activated HSCs on HCC, HSC cell line LX-2, which shows properties of activated HSC was used to test for its ability to influence HCC cell lines Hep3B and HepG2. Interestingly, up-regulation of invasive and migratory ability was found in Hep3B cells as a consequence of LX-2 cultured media (CM) exposure, but not in HepG2 cells (). Recently studies have proved that epithelial mesenchymal transition (EMT) participates in tumor metastasis. We subsequently examined expression of EMT-related molecules. According to real time PCR () and western blot () analysis, after 24 h LX-2 CM treatment of Hep3B, epithelial marker E-cadherin expression levels were significantly decreased, and mesenchymal marker Vimentin expression levels were remarkably increased. Consistently, such circumstances were not aroused in HepG2 cells (). Furthermore, immunofluorescence staining also showed that E-cadherin was degraded and Vimentin was induced in the cytoplasm of Hep3B cells upon LX-2 CM exposure (). Thus, LX-2 cells promoted Hep3B cells invasion and migration by inducing EMT occurrence.
Figure 1. LX-2 cells promoted invasion and migration of Hep3B cells, but not HepG2 cells. A. Invasiveness of cells was determined using the Transwell assay. Hep3B and HepG2 cells treated with LX-2 cells CM were plated in the upper chamber of the Transwell and allowed to grow for 24 hours in serum-free medium, 5% fetal bovine serum was placed in the lower chamber. The number of cells that invaded through the Matrigel was counted in 10 fields under the ×20 objective lens, and is shown as the mean ± standard deviation. Scale bar = 100μm. **P < 0.01, NS, no significant. B. Migration of cells was determined using the Transwell assay. Hep3B and HepG2 cells treated with LX-2 cells CM were plated in the upper chamber of the Transwell and allowed to grow for 24 hours in serum-free medium, 5% fetal bovine serum was placed in the lower chamber. The number of cells that migrated was counted in 10 fields under the ×20 objective lens, and is shown as the mean ± standard deviation. Scale bar = 100μm. ***P < 0.001, NS, no significant. C. Real-time PCR was used to detect E-cadherin and Vimentin expression in Hep3B and HepG2 cells treated with LX-2 cells CM, GAPDH was used as an internal reference. **P < 0.01, NS, no significant. D. Western-blot was used to detect E-cadherin and Vimentin expression in Hep3B and HepG2 cells treated with LX-2 cells CM, GAPDH was used as an internal reference. E. Immunofluoresence was used to evaluate E-cadherin and Vimentin expression in Hep3B and HepG2 cells treated with LX-2 cells CM. Scale bar = 100 μm.
HGF secreted by LX-2 cells was capable of promoting Hep3B cells invasion and migration
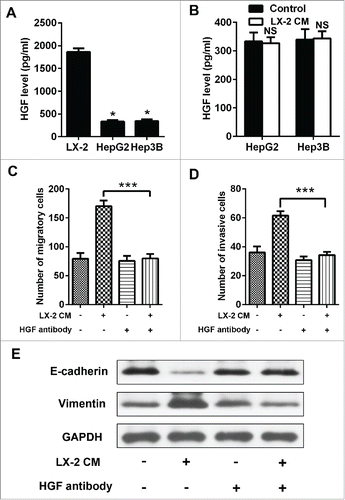
To identify whether HGF which is secreted by LX-2 cells promoted Hep3B cells invasion, we measured HGF levels in cultured media from LX-2 cells. We observed that HGF levels continued to rise for 24h (). We also measured HGF levels in cultured media of Hep3B and HepG2 cells at 24h. Both cells virtually secreted HGF at a low level (). Additionally, we found that presence of HGF neutralizing antibody decreased the migration and invasion of Hep3B cells when treated with LX-2 cells-derived HGF (). And EMT phenotype was also attenuated (). In summary, these results indicated that LX-2 cells-derived HGF could promote Hep3B cells invasion and migration.
Figure 2. HGF secreted by LX-2 cells was capable of promoting Hep3B cells invasion and migration. A. To assess HGF levels in Hep3B, HepG2 cells and LX-2 cells CM by ELISA. *P < 0.01. B. To assess HGF levels in Hep3B and HepG2 cells treated with LX-2 cells CM by ELISA. NS, no significant. C and D. Hep3B cells treated with LX-2 cells CM and HGF antibody, then the number of invasive and migratory cells was counted by transwell assay. ***P < 0.001. E. Western-blot was used to detect E-cadherin and Vimentin expression in Hep3B cells treated with LX-2 cells CM and HGF antibody, GAPDH was used as an internal reference.
Met-dependent migration and invasion were regulated by p53
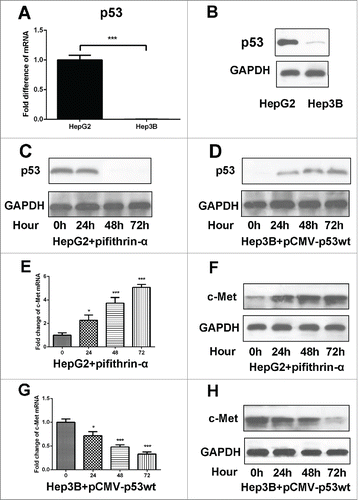
Met is a receptor protein tyrosine kinase activated by HGF, which is a crucial determinant of metastatic progression. We next interrogated whether there was a discrepancy in the level of c-Met expression between Hep3B and HepG2 cells to come out different migratory and invasive ability upon LX-2 CM treatment. Comparatively, c-Met mRNA and protein levels were particularly higher in Hep3B than HepG2 cells (). Then a c-Met receptor tyrosine kinase inhibitor, PHA665752 was used to target HGF/c-Met, the invasion and migration of Hep3B cells induced by LX-2 CM were efficiently blocked (). And EMT phenotype was also attenuated (). Previous studies have identified that p53 played as an important regulator of c-Met expression. We then detected the expression of p53 in Hep3B and HepG2 cells. The results showed that p53 was deficiency in Hep3B cells (). We used p53 inhibitor pifithrin-α (Beyotime, S1816) to abate endogenous p53 expression in HepG2 cells () and transfected with pCMV-p53wt to induce ectopic p53 expression in Hep3B cells (). Then we found that c-Met expression levels continued to rise for 72h in HepG2 cells (), and kept on declining in Hep3B cells (). These results demonstrated that c-Met-dependent migration and invasion in Hep3B cells were regulated by p53.
Figure 3. c-Met overexpression contributed to the migration and invasion of Hep3B cells. A and B. Real-time PCR and western-blot were used to detect c-Met expression in Hep3B and HepG2 cells, GAPDH was used as an internal reference. ***P < 0.001. C and D. Hep3B cells treated with LX-2 cells CM and c-Met inhibitor PHA665752, then the number of invasive and migratory cells was counted by transwell assay. ***P < 0.001. E. Western-blot was used to detect E-cadherin and Vimentin expression in Hep3B cells treated with LX-2 cells CM and c-Met inhibitor PHA665752, GAPDH was used as an internal reference.
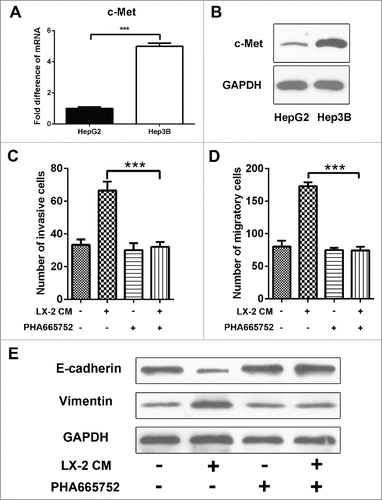
Figure 4. c-Met expression was regulated by p-53. A and B. Real-time PCR and western-blot were used to detect c-Met expression in Hep3B and HepG2 cells, GAPDH was used as an internal reference. ***P < 0.001. C. Western-blot was used to detect p53 expression in HepG2 cells treated with p53 inhibitor pifithrin-α. D. Western-blot was used to detect p53 expression in Hep3B cells transfected with pCMV-p53wt to induce ectopic. E and F. Real-time PCR and Western-blot were used to detect c-Met expression in HepG2 cells treated with p53 inhibitor pifithrin-α. *P < 0.01, ***< 0.001. G and H. Real-time PCR and Western-blot were used to detect c-Met expression in Hep3B cells transfected with pCMV-p53wt to induce ectopic p53 expression. *P < 0.01, ***< 0.001.
Activated HGF/c-Met signaling pathway upregulated snail expression
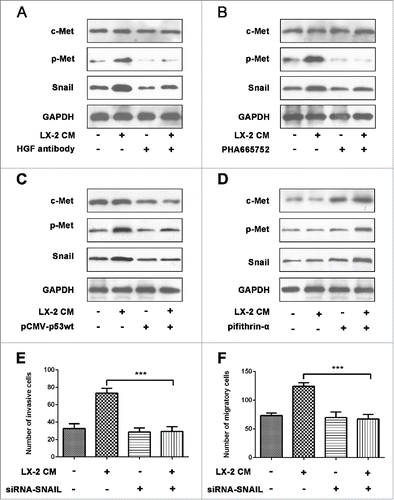
Snail transcriptional repressor is considered as an essential regulator of EMT.Citation19 Upon LX-2 CM exposure, phosphorylated Met (p-Met) expression in Hep3B cells was down-regulated when HGF inhibitor presented. Simultaneously, the mode of Snail expression was analogous—drastically decreased in Hep3B cells (). By application of PHA665752, c-Met activation was adequately suppressed and p-Met was in low level as well as Snail expression (). In combination with the transwell assays, these results suggested that HGF/c-Met signaling pathway promoted HCC cells invasion and migration presumably by increasing expression of Snail. Additionally, pCMV-p53wt was added in Hep3B cells to induce p53 expression, we found that c-Met expression was suppressed, p-Met and Snail expression was also decreased (). On the contrary, p53 expression was inhibited by pifithrin-α in HepG2 cells, then c-Met expression was induced, the levels of p-Met and Snail were also increased (). To identify whether Snail activation was necessary for the invasion of Hep3B cells which was induced by HGF/c-Met signaling, we used Snail siRNA to down-regulate its expression. The data showed that the invasive and migratory capacity was adequately suppressed when Snail siRNA presented (). Results above indicated that HGF/c-Met signaling promoted HCC cells invasion and migration by up-regulating Snail expression.
Figure 5. Activated HGF/c-Met signaling pathway upregulated Snail expression. A and B. Western-blot were used to detect c-Met, p-Met and Snail expression in Hep3B cells treated with LX-2 CM, HGF antibody and PHA665752, respectively. GAPDH was used as an internal reference. C. Western blot was used to detect c-Met, p-Met and Snail expression in Hep3B cells transfected with pCMV-p53wt to induce ectopic p53 expression. GAPDH was used as an internal reference. D. Western-blot were used to detect c-Met, p-Met and Snail expression in HepG2 cells treated withp53 inhibitor pifithrin-α. GAPDH was used as an internal reference. E and F. Hep3B cells treated with LX-2 cells CM and Snail siRNA, then the number of invasive and migratory cells was counted by transwell assay. ***P < 0.001.
Discussion
The activation of MET has been particularly associated with metastatic behavior of tumors.Citation20 MET is a receptor tyrosine kinase (RTK) that has a role in various cellular processes, including proliferation, invasion and cell scattering.Citation21 And we showed here that HCC cell lines in response to HGF in a Met-dependent manner which regulated by p53. The results suggested that Hep3B cells with p53 deficiency harbored the invasive feature in response to HGF, and HepG2 cells with wild-type p53 expression did not show the phenotype. Additionally, we found that HGF/c-Met signaling pathway promoted cell migration and invasion through Snail activation.
HGF/c-Met axis dysregulation occurs in a variety of solid tumors and haematopoietic derived malignancies, which plays a key role in malignant transformation by promoting tumor cell migration, epithelial to mesenchymal transition, and invasion.Citation22 In our work, HCC cell line Hep3B displayed invasion and migration as well as EMT phenotype when treated with HGF secreted by LX-2 cells, and the phenotype was weaken when HGF antibody was presence. Met activation by HGF can induce cell scattering, invasion, thereby acting as a powerful expedient for cancer dissemination.Citation23 In this study, Hep3B cell line treated with selective c-Met inhibitor PHA665752 resulted in decreased invasive capacity and EMT phenotype in vitro. These data suggested that HGF and c-MET may be promising targets in the treatment of HCC and c-MET overexpression may be a predictive biomarker of response.
The intersection of p53 and Met signaling has been proposed by several previous studies that demonstrated an ability of wild-type p53 function to control Met expression.Citation24 And complete lack of p53 abolishes both mechanisms of Met regulation, leading to its maximal expression and metastasis-related cancer traits, such as cell motility and invasion.Citation25 In our work, 2 HCC cell lines were mentioned, Hep3B and HepG2 cell lines. When HCC cell lines were treated with HGF, Hep3B cells showed invasive characteristic and EMT phenotype, however HepG2 cell lines did not display the phenotype. Then we found that Hep3B cells harbored p53 loss and c-Met overexpression, and HepG2 cells had wild-type p53 and low c-Met expression. It may decide the different invasive capacity in these 2 HCC cell lines when treated with HGF. It has been reported that patients with p53-null HCC had worse prognosis than those with other p53 status in their cancers.Citation26-28 Notably, Met over-expression has been reported to be associated with poor prognosis of patients with HCC as well.Citation29 Therefore, our observations that p53 had a feedforward loop regulation of Met expression may provide a mechanistic link between 2 independently reported prognostic factors, p53-null status and Met overexpression.
Snail protein function and gene expression are regulated by various mechanisms. For example, Snail expression in epithelial cells can be induced by TGFβ, oncogenic Ras, or HGF.Citation30-32 HGF induces a rapid and transient increase in both Snail mRNA and protein levels. Thereby, EMT occurred. A similar correlation has been previously reported in HCC cells, where Snail over-expression and E-cadherin downregulation were associated with higher invasiveness.Citation33 Present study showed that Snail expression was decreased when HGF antibody was presence. Additionally, when HCC cells were treated with c-Met inhibitor PHA665752 and p53 inhibitor pifithrin-α, Snail expression was also decreased. And the HGF/c-Met signaling pathway involved invasive and migratory capacity of Hep3B cells was attenuated when Snail siRNA was used. It meant that HGF/c-Met signaling induced Snail upregulation, which contributed to the cell invasion and migration.
In conclusion, our data suggested the HGF/c-MET signaling played an important role in the HCC cells invasion and migration. And loss of p53 expression enhanced the phenomena. HGF/c-Met signaling promoted Snail expression, which lead to the HCC cells invasion and migration.
Materials and methods
Cell culture
Human HCC lines Hep3B and HepG2 were incubated in Dulbecco’s Modified Eagle Medium (DMEM, Gibco-BRL, Gaithersburg, MD, USA) containing 10% FBS and antibiotics (100mg/L penicillin and 100mg/L streptomycin); human immortal hepatic stellate cell line LX-2 was maintained in RPMI 1640 (Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10% FBS and antibiotics (100mg/L penicillin and 100mg/L streptomycin) in a humidified incubator under 5% CO2 at 37°C.
Conditioned medium and ELISA
Cultured media (CM) were collected from LX-2 cells cultured in 2D culture dishes and centrifuged at 1000g for 5 minutes to obtain the supernatant. The levels of HGF in CM were determined by using human HGF ELISA kit (HY10158E, Shanghai HengYuan Biological Technology Co., Ltd) according to manufacturer’s instructions.
Transwell invasion assay
The invasive ability of tumor cells was performed in vitro using a transwell chamber system with 8.0μm pore polycarbonate filter inserts (Corning Coster, Cambridge, MA, USA). The lower side of the filter was coated with 10μL gelatin (1mg/ml), and the upper side was coated with 10μl of the matrigel. Tumor cells (5×10 3) suspended in 200μl serum free DMEM were seeded in the upper part of the filter and 500μl of DMEM media containing 10% FBS was added the lower compartment. After 48h of incubation at 37°C under 5% CO2, the upper surface of the membrane was scrubbed with a cotton swab and the cells in the lower membrane were fixed with 4% paraformaldehyde and stained with crystal violet. The number of invasive cells was showed as the average of 5 random fields under the microscope at×200 magnification.
Transwell migration assay
The transwell migration assay was performed using Transwell migration chambers (8.0μm, not containing gelatin and matrigel). The remaining steps were similar to the transwell invasion assay.
Real time PCR
Total RNA was obtained by using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. Real time PCR was performed with Real-time PCR systems adopted 10 microliters system (5μl SYBY green, 1μl forward and reverse specific primers, respectively, 2μl cDNA and 1µl ddH2O) at the condition of 95°C for 10min, followed by 40 cycles of 95°C for 15s, 60°C for 30s and 72°C for 30s and ended with 95°C for 1 min, 55°C for 30s, 95°C for 30s.
Western blot
Protein samples were collected directly by cell extraction buffer (Beyotime, P0013) containing a protease inhibitors PMSF (Cwbiotech, CW0037). The equivalent aliquots of protein were eletrophoresed on a 10% SDS/polyacrylamide gel in 1XTris-glycin buffer and transferred to nitrocellulose membranes and incubated with primary antibodies overnight at 4°C. Following incubated with secondary antibody 1h at room temperature. The immunoreactive proteins were detected by enhanced Chemoluminescence Substrate, and the blot was scanned and densitometric analysis with Image J software. The primary antibodies used in our experiment including: E-cadherin (ab53033, diluted 1:1000), Vimentin (ab135708, diluted 1:1000), c-Met (ab47606, diluted 1:500), p53 (ab31333, diluted 1:500), p-Met (CST, #3077, diluted 1:1000), Snail (R&D systems, AF3639, diluted 1: 500) and GAPDH (ab9385, diluted 1:5000).
Immunofluorescence
After treatment, cells in 24 well plate were fixed with 4% paraformaldehyde and incubated with E-cadherin (diluted 1:200) or Vimentin (diluted 1:200) overnight at 4°C. And then incubated with Alexa Fluor 488-labeled secondary antibodies (Molecular Probes, Invitrogen, Paisley, UK, diluted 1:200) for 1h at 37°C. DAPI (Sigma-Aldrich) was used to stain the nuclei. Fluorescence intensity was evaluated by using a confocal microscope (Leica TCS SP2).
Disclosure of potential conflicts of interest
The authors declare that they have no conflicts of interest to disclose.
Funding
This project was supported by the Key Basic Research Project of China (Grant NO. 2012CBA01303); National Natural Science Foundation of China (Grant No. 81372312, 81472737, 81402018, 81402020, 81401308, 81402026, 81372330, 81572444, 81502417, 81502543, 81221061); Special Funds for National Key Sci-Tech Sepcial Project of China (Grant No. 2016ZX10002019-005-002); Shanghai Science and Technology Committee (Grant No. 14ZD1900403, 14ZR1409200, 15PJ1410600); Shanghai Municipal Education Commission (Grant No. 14ZZ086).
References
- Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004; 127:S5-S16; PMID:15508102; http://dx.doi.org/10.1053/j.gastro.2004.09.011
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a Cancer Journal for Clinicians 2011; 61:69-90; PMID:21296855
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 2006; 6:674-87; PMID:16929323; http://dx.doi.org/10.1038/nrc1934
- Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008; 48:1312-27; PMID:18821591; http://dx.doi.org/10.1002/hep.22506
- Thompson AI, Conroy KP, Henderson NC. Hepatic stellate cells: central modulators of hepatic carcinogenesis. BMC Gastroenterol 2015; 15:63; PMID:26013123; http://dx.doi.org/10.1186/s12876-015-0291-5
- Carloni V, Luong TV, Rombouts K. Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: more complicated than ever. Liver Int 2014; 34:834-43; PMID:24397349; http://dx.doi.org/10.1111/liv.12465
- Amann T, Bataille F, Spruss T, Muhlbauer M, Gabele E, Scholmerich J, Kiefer P, Bosserhoff AK, Hellerbrand C. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci 2009; 100:646-53; PMID:19175606; http://dx.doi.org/10.1111/j.1349-7006.2009.01087.x
- Yu G, Jing Y, Kou X, Ye F, Gao L, Fan Q, Yang Y, Zhao Q, Li R, Wu M, et al. Hepatic stellate cells secreted hepatocyte growth factor contributes to the chemoresistance of hepatocellular carcinoma. PloS one 2013; 8:e73312; PMID:24023859; http://dx.doi.org/10.1371/journal.pone.0073312
- Jeffers M, Fiscella M, Webb CP, Anver M, Koochekpour S, Vande Woude GF. The mutationally activated Met receptor mediates motility and metastasis. Proc Natl Acad Sci U S A 1998; 95:14417-22; PMID:9826715; http://dx.doi.org/10.1073/pnas.95.24.14417
- Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 1991; 67:901-8; PMID:1835669; http://dx.doi.org/10.1016/0092-8674(91)90363-4
- Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol 1995; 129:1177-80; PMID:7775566; http://dx.doi.org/10.1083/jcb.129.5.1177
- Kolatsi-Joannou M, Moore R, Winyard PJ, Woolf AS. Expression of hepatocyte growth factor/scatter factor and its receptor, MET, suggests roles in human embryonic organogenesis. Pediatric Res 1997; 41:657-65; PMID:9128288; http://dx.doi.org/10.1203/00006450-199705000-00010
- Wang Y, Selden C, Farnaud S, Calnan D, Hodgson HJ. Hepatocyte growth factor (HGF/SF) is expressed in human epithelial cells during embryonic development; studies by in situ hybridisation and northern blot analysis. J Anatomy 1994; 185(Pt 3):543-51; PMID:7649790
- Cao HH, Cheng CY, Su T, Fu XQ, Guo H, Li T, Tse AK, Kwan HY, Yu H, Yu ZL. Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol Cancer 2015; 14:103; PMID:25971889; http://dx.doi.org/10.1186/s12943-015-0367-4
- Hwang CI, Matoso A, Corney DC, Flesken-Nikitin A, Korner S, Wang W, Boccaccio C, Thorgeirsson SS, Comoglio PM, Hermeking H, et al. Wild-type p53 controls cell motility and invasion by dual regulation of MET expression. Proc Natl Acad Sci U S A 2011; 108:14240-5; PMID:21831840; http://dx.doi.org/10.1073/pnas.1017536108
- Tepper CG, Gregg JP, Shi XB, Vinall RL, Baron CA, Ryan PE, Desprez PY, Kung HJ, deVere White RW. Profiling of gene expression changes caused by p53 gain-of-function mutant alleles in prostate cancer cells. Prostate 2005; 65:375-89; PMID:16037992; http://dx.doi.org/10.1002/pros.20308
- Di Agostino S, Strano S, Emiliozzi V, Zerbini V, Mottolese M, Sacchi A, Blandino G, Piaggio G. Gain of function of mutant p53: the mutant p53/NF-Y protein complex reveals an aberrant transcriptional mechanism of cell cycle regulation. Cancer Cell 2006; 10:191-202; PMID:16959611; http://dx.doi.org/10.1016/j.ccr.2006.08.013
- O'Farrell TJ, Ghosh P, Dobashi N, Sasaki CY, Longo DL. Comparison of the effect of mutant and wild-type p53 on global gene expression. Cancer Res 2004; 64:8199-207; PMID:15548685; http://dx.doi.org/10.1158/0008-5472.CAN-03-3639
- de Herreros AG, Peiro S, Nassour M, Savagner P. Snail family regulation and epithelial mesenchymal transitions in breast cancer progression. J Mammary Gland Biol Neoplasia 2010; 15:135-47; PMID:20455012; http://dx.doi.org/10.1007/s10911-010-9179-8
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003; 4:915-25; PMID:14685170; http://dx.doi.org/10.1038/nrm1261
- Stoker M, Perryman M. An epithelial scatter factor released by embryo fibroblasts. J of Cell Sci 1985; 77:209-23; PMID:3841349
- Tavian D, De Petro G, Benetti A, Portolani N, Giulini SM, Barlati S. u-PA and c-MET mRNA expression is co-ordinately enhanced while hepatocyte growth factor mRNA is down-regulated in human hepatocellular carcinoma. Int J Cancer J Int du Cancer 2000; 87:644-9; PMID:10925356; http://dx.doi.org/10.1002/1097-0215(20000901)87:5%3c644::AID-IJC4%3e3.0.CO;2-W
- Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Dis 2008; 7:504-16; PMID:18511928; http://dx.doi.org/10.1038/nrd2530
- Seol DW, Chen Q, Smith ML, Zarnegar R. Regulation of the c-met proto-oncogene promoter by p53. J Biol Chem 1999; 274:3565-72; PMID:9920903; http://dx.doi.org/10.1074/jbc.274.6.3565
- Hwang CI, Choi J, Zhou Z, Flesken-Nikitin A, Tarakhovsky A, Nikitin AY. MET-dependent cancer invasion may be preprogrammed by early alterations of p53-regulated feedforward loop and triggered by stromal cell-derived HGF. Cell Cycle 2011; 10:3834-40; PMID:22071625; http://dx.doi.org/10.4161/cc.10.22.18294
- Hsu HC, Peng SY, Lai PL, Sheu JC, Chen DS, Lin LI, Slagle BL, Butel JS. Allelotype and loss of heterozygosity of p53 in primary and recurrent hepatocellular carcinomas. A study of 150 patients. Cancer 1994; 73:42-7; PMID:7506118; http://dx.doi.org/10.1002/1097-0142(19940101)73:1%3c42::AID-CNCR2820730109%3e3.0.CO;2-D
- Hsu HC, Tseng HJ, Lai PL, Lee PH, Peng SY. Expression of p53 gene in 184 unifocal hepatocellular carcinomas: association with tumor growth and invasiveness. Cancer Res 1993; 53:4691-4; PMID:8402647
- Liu SH, Lin CY, Peng SY, Jeng YM, Pan HW, Lai PL, Liu CL, Hsu HC. Down-regulation of annexin A10 in hepatocellular carcinoma is associated with vascular invasion, early recurrence, and poor prognosis in synergy with p53 mutation. Am J Pathol 2002; 160:1831-7; PMID:12000734; http://dx.doi.org/10.1016/S0002-9440(10)61129-7
- Chu JS, Ge FJ, Zhang B, Wang Y, Silvestris N, Liu LJ, Zhao CH, Lin L, Brunetti AE, Fu YL, et al. Expression and prognostic value of VEGFR-2, PDGFR-beta, and c-Met in advanced hepatocellular carcinoma. J Exp Clin Cancer Res 2013; 32:16; PMID:NOT_FOUND; http://dx.doi.org/10.1186/1756-9966-32-16
- Tan C, Costello P, Sanghera J, Dominguez D, Baulida J, de Herreros AG, Dedhar S. Inhibition of integrin linked kinase (ILK) suppresses beta-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC−/− human colon carcinoma cells. Oncogene 2001; 20:133-40; PMID:11244511; http://dx.doi.org/10.1038/sj.onc.1204052
- Gotzmann J, Huber H, Thallinger C, Wolschek M, Jansen B, Schulte-Hermann R, Beug H, Mikulits W. Hepatocytes convert to a fibroblastoid phenotype through the cooperation of TGF-beta1 and Ha-Ras: steps towards invasiveness. J Cell Sci 2002; 115:1189-202; PMID:11884518
- Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J 2006; 25:3534-45; PMID:16858414; http://dx.doi.org/10.1038/sj.emboj.7601213
- Sugimachi K, Tanaka S, Kameyama T, Taguchi K, Aishima S, Shimada M, Sugimachi K, Tsuneyoshi M. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin Cancer Res 2003; 9:2657-64; PMID:12855644
