ABSTRACT
Objective: Cardiosphere-derived cells (CDCs) improve cardiac function and attenuate remodeling in ischemic and non-ischemic cardiomyopathy, and are currently obtained through myocardial biopsy. However, there is not any study on whether functional CDCs may be obtained through cadaveric autopsy with similar benefits in non-ischemic cardiomyopathy. Methods: Cardiac tissues from human or mouse cadavers were harvested, plated at 4°C, and removed at varying time points to culture human CDCs (CLH-EDCs) and mouse CDCs (CM-CDCs). The differentiation and paracrine effects of CDCs were also assessed. Furthermore, intramyocardial injection of cadaveric CM-CDCs was performed in an induced dilated cardiomyopathy (DCM) model. Results: With the extension of post mortem hours, the number of CLH-EDCs and CM-CDCs harvested from autopsy specimens decreased. The expressions of von Willebrand factor (VWF) and smooth muscle actin (SMA) on CDCs were gradually reduced, however, cardiac troponin I (TNI) expression increased in the 24 h group compared to the 0 h group. CLH-EDCs were also found to have similar paracrine function in the 24 h group compared to 0 h group. 8 weeks after CM-CDCs transplantion to the injured heart, mean left ventricular ejection fraction increased in both 0 h (64.99 ± 3.4%) and 24 h (62.99 ± 2.8%) CM-CDCs-treated groups as compared to the PBS treated group (53.64 ± 5.6 cm), with a decrease in left ventricular internal diastolic diameter (0.29 ± 0.08 cm and 0.32 ± 0.04 cm in 0 h and 24 h groups, vs. 0.41 ± 0.05 cm in PBS group). Conclusion: CDCs from cadaveric autopsy are highly proliferative and differentiative, and may be used as a source for allograft transplantation, in order to decrease myocardial fibrosis, attenuate left ventricular remodeling, and improve heart function in doxorubicin-induced non-ischemic cardiomyopathy.
Introduction
Studies have shown that cardiac stem cell transplantation improves cardiac function not only in ischemic cardiomyopathy, but also in non-ischemic dilated cardiomyopathy.Citation1 However, this promising therapeutic strategy has been restricted by the lack of source donor cells. Hence, scientists tried finding a new cell source to solve current problem. Pioneer studies have confirmed that non-heart-beating donors have been considered as a source of transplantable stem cells. Mesenchymal stem cells (MSCs), skeletal muscle stem cells, and hepatic stem cells could be cultured from cadaveric organs and tissues while maintaining viability.Citation2-4 Successful isolation, propagation, functional characterization, and cryopreservation of human cadaveric MSCs derived from variously sized arterial segments 4 d post mortem have been maintained for more than 5 y.Citation2 Latilet et al.Citation3 confirmed that viable and functional skeletal myogenic cells can be obtained from humans up to 17 days, and from mice up to 14 d post mortem. Therefore, we hypothesized that cadaver post mortem may be a new source of stem cells.
Cardiosphere-derived cells (CDCs) were first shown by Messina et al.Citation5 to be clonally expanded from mouse and human myocardial biopsies. CDCs, as their name implies, are created from self-assembling heart-derived multicellular clusters which express Sca-1, c-kit, Flk, and CD31.Citation6 Currently, transplantable CDCs are isolated only from surgical and myocardial biopsy resection specimens. The mechanisms by which CDCs improve cardiac function include: (a) paracrine function; (b) multilineage differentiation; and (c) activation of endogenous cardiac stem cells.Citation7-8 CDCs demonstrated stronger and more balanced paracrine function than bone marrow-derived MSCs, adipose tissue-derived MSCs, and bone marrow mononuclear cells.Citation9 Furthermore, allogeneic CDC transplantation without immunosuppression induces only a transient mild local immune reaction in a rat myocardial infarction (MI) model.Citation10-11 The CADUCEUS (CArdiosphere-Derived aUtologous Stem CElls to Reverse ventricUlar dySfunction) trial proved the efficacy and safety of CDC transplantation for treatment of MI.Citation12 Recently, Mohammad et al. found that CDC treatment prevents functional deterioration, attenuates oxidative stress, promotes cardiomyocyte proliferation, and reduces mortality in a transgenic mouse model of dilated cardiomyopathy (DCM).Citation13
We are the first to our knowledge to demonstrate that there are still a large number of surviving cardiac stem cells in the cadaveric heart, and that a sufficient amount of functional CDCs may be obtained at autopsy. In vitro, we confirmed that CDCs derived from cadaveric tissues have differentiation potential. We further evaluated the potential role for CDCs on growth factor secretion, and demonstrated that CDCs could secrete large amounts of growth factors in all groups. We therefore tested the concept that CDCs could be useful in a mouse model of doxorubicin-induced non-ischemic cardiomyopathy.
Methods
Ethical approval
Human samples were collected according to guidelines of the Ethical Committee of Harbin Medical University after informed consent in an institutional review board approved protocol. All experimental animal procedures were approved by the Local Ethical Committee of Harbin Medical University for Animal Care and Use.
Cell culture
Human myocardial tissue was derived from atrial or ventricular biopsy specimens of patients aged 3 to 70 y who were undergoing heart surgery (Table S1). Because obtaining post mortem tissues from human is difficult, the fresh human myocardial tissues were removed, and plated at 4°C for different durations (0 h, 24 h, 72 h, 120 h) to simulate cadaveric environment. All C57BL/6 mice were obtained from the laboratory animal science department of the Second Affiliated Hospital of Harbin Medical University (Heilongjiang, China). Some CM-CDCs were used to culture in vivo, the others were used to inject into DCM mouse hearts. Cardiosphere and CDCs were isolated from human and mouse tissues using a previously published method.Citation14 Detailed methods are available in the Data Supplement.
Cell counting kit-8 (CCK8) assay
Mouse explant-derived cells (CM-EDCs) and CM-CDCs were plated in 96-well plates at a density of 4,000 cells per well with 100 μL of complete culture medium. After adhesion for 24 h, cells were cultured for another 1, 3, 5, 7, and 9 day. Wells in which only culture medium were added served as blanks. At each time point, the supernatant was removed and 100 μL of DMEM medium (HyClone, Logan, UT, USA) containing 10 μL of CCK8 (Dojindo, Kumamoto, Japan) were added to each well for 1 h at 37° C. Absorbance was recorded at 450 nm. All experiments were independently repeated at least 3 times.
Immunofluorescence
To characterize CDCs among isolated cells, CDCs were fixed with 1% formaldehyde for 30 min. After washing with PBS, cells were blocked with 5% BSA, and incubated at 37° C for 1 h with human anti-GATA4 antibody (1:200, ab84593, Abcam Ltd., Cambridge, MA, USA), human anti-Nkx2.5 antibody (1:200, ab97355, Abcam), human anti-cardiac Troponin I antibody (TNI) (1:100, ab47003, Abcam), human anti-von Willebrand factor antibody (VWF) (1:100, sc-14014, Santa, St-Louis, MO, USA) and human anti-smooth muscle antibody(SMA) (1:100, ab5694, Abcam). Cells were then washed and incubated in the dark for 2 h at 37° C with goat anti-rabbit IgG (H+L) antibodies (1:200, ZSGBBIO, Beijing, China). After washing, nuclei were counterstained with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI, Beyotime, Jiangsu, China). Cells were examined under a fluorescent microscope (DMI4000B, Leica, Germany).
Flow cytometry
A single cell suspension of 0.5–1.0 × 106 cells/ml in PBS (Ca+2/Mg+2free), were incubated in the dark at 4°C for 30 min for tagging with the following fluorescent primary antibodies: anti-mouse anti-mouse CD117-FITC (eBiosciences), anti-mouse Sca-1-FITC (BD Biosciences), anti-mouse CD133-PE (eBiosciences), anti-human CD117-FITC (eBiosciences), and anti-human CD105-PE (eBiosciences), anti-human CD90-PE (eBiosciences), anti-human CD31-PE (eBiosciences). A total of 10,000 events were acquired using a FACS Canto II system (BD Biosciences). Flow cytometry was carried out using cells from 3 independent experiments and was performed in duplicate.
Differentiation CDC potential in vitro
For differentiations of cardiomyocyte, endothelial cell, and smooth muscle cell, CDCs were treated with 5-azacytidine (10 μmol/L, Shanghai, China), vascular endothelial growth factor (10 ng/ml, Gibco, Grand Island, NY, USA), and platelet-derived growth factor-BB (5 ng/ml, Miltenyi Biotec, Bergisch Gladbach, Germany) plus human transforming growth factor β1 (2.5 ng/ml, Miltenyi Biotec), respectively. Then, the cells were stained for cardiac troponin I (TNI), von Willebrand factor (VWF) and smooth muscle actin (SMA).
Quantitative real-time RT-PCR
Total RNA was extracted from cells using a PureLink RNA Mini Kit (Life Technologies) according to the manufacturer's protocol; 2 µg of total RNA was used for cDNA. RT-PCR was performed using SYBR Premix Ex Taq II (Tiangen, Beijing, China) and 1 μg purified RNA for a total volume of 20 μl. All primers for RT-PCR were designed with Primer Express software and synthesized by Sigma-Genosys Japan (Tokyo, Japan). The human and mouse primers used for RT-PCR were shown in Table S2.
Enzyme-linked immunoadsorbent assay
To compare growth factor production potency, insulin-like growth factor-1 (IGF-1), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF) were measured with enzyme-linked immunoadsorbent assay (ELISA) kits, according to the manufacturer's instructions (BlueGene, Shanghai, China). Growth factors in conditioned media were measured using ELISA.
Animal model and treatments
Chronic cardiomyopathy was induced in 8–12 week old C57BL/6 male mice by receiving intraperitoneal injection of 4 mg/kg doxorubicin (Sigma, St-Louis, MO, USA) for 6 times on alternate days. Control mice received equal volumes of saline injections. Four weeks after the last doxorubicin injection, mice were randomly treated by intramyocardial (left ventricular free wall) injection of one of the following, using a 30 gauge needle at four time points: 50 µL PBS (control group, n = 10), 5 × 105 0 h-CM-CDCs (n = 8), 5 × 105 24 h-mCDC (n = 8), or 5 × 105 72 h-CM-CDCs (n = 8).
Echocardiography
A baseline echocardiogram was performed on 8–12 week old mice using a GE/Vingmed ultrasound Vivid7 (GEHealthcare, Little Chalfont, UK). In DCM mice, echocardiography was performed 6 weeks after intraperitoneal injection of doxorubicin. Echocardiography was performed 8 weeks after cell transplantation in model mice. Details and parameters of echocardiography were shown in Table S3.
Histological examination
Hearts of mice were removed and studied histologically. Hematoxylin and Eosin (HE) staining was assessed by NIH ImageJ software for morphometric parameters. To detect fibrosis in cardiac muscle, the LV myocardium was fixed in 10% formalin, cut transversely, paraffin-embedded, and stained with Masson's trichrome.
Statistical analysis
Statistical analysis was performed independently. All results are presented as the mean ± standard error of the mean except as noted. Data sets were first tested for normality and variance. If both were assured, statistical significance was determined by one-way analysis of variance. If either normality or variance tests failed, nonparametric tests were used. A p-value of less than 0.05 was considered statistically significant.
Results
Viability of human and mouse stem cells at different time points
We first investigated whether viable CDCs could be isolated post-mortem. Donor human and mouse tissues were maintained at 4° C until cell isolation. The isolation of viable EDCs from humans was performed up to 120 h, and in mice up to 72 h post mortem (). As time progressed after death, fewer cells could be harvested. Histologic examination of human cardiac biopsies showed severe autolytic alterations with edema in the 24 and 72 h groups. Nuclear pyknosis and autolytic alterations were more significant in the 120 h group (). Similar results were obtained at 0–72 h in mice heart tissue post mortem (). With the extension of post mortem hours, the number of EDCs harvested after autopsy gradually decreased (), and EDCs required more time to start growing ().
Figure 1. Viability of human and mouse cardiosphere-derived cells (CDCs) post mortem. Human heart and mouse cadaver tissue were plated at 4° C, and removed at different time points for HE staining and for culturing CDCs. Hearts of mice were fixed with 4% paraformaldehyde, and then were paraffin-embedded and cut transversely into sections. These sections were stained with hematoxylin and eosin (HE). (A-D) Representative images of CLH-EDCs (A) and CM-EDCs (C) after 8 d in culture, and representative HE staining images of human (B) and mouse (D) heart (C scale bar = 50 µm; A, B, D scale bar = 100 µm). (E and F) Representative CM-EDCs (E) and CLH-EDCs (F) were harvested from autopsy specimens on one plate. (G and H) Representative time of CM-EDCs (G) and CLH-EDCs (H) growth from autopsy specimens. (I and J) Representative proliferation of CM-EDCs (I) and CM-CDCs (J) were determined by CCK-8 every 2 d for 9 d.
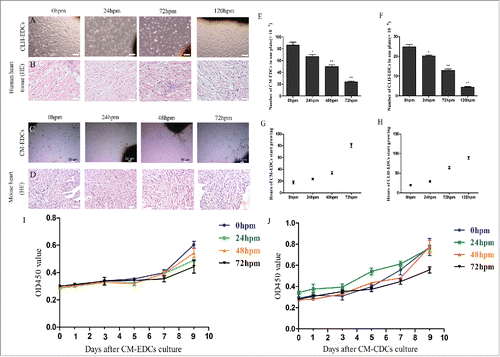
We quantified the proliferative ability of CM-EDCs and CM-CDCs using a CCK-8 assay. mEDC start proliferate after 5 d of culture, and proliferate actively until 9 d. But mCDC started to grow gradually from 1 day to 9 d. Cell proliferation was inhibited in the 72 h group of CM-EDCs and CM-CDCs in comparison with the 0 hour group ().
Characteristics of CDCs derived from mice and humans
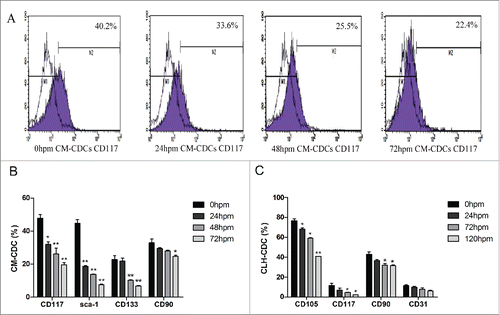
Flow cytometry was performed to characterize the antigenic profile of CDCs from mice and humans. In CM-CDCs, the expressions of CD117 and sca-1 were decreased in 24 h groups compared with 0 h groups, while there were no significant changes for the expressions of CD133 and CD90 (). For CLH-EDCs, no statistical differences in CD117, CD90 and CD31 expression were found between 0 h and 24 h groups, however, CD105 expression was decreased ().
Figure 2. Characteristics of CDCs derived from mouse and human. (A) CD117 expression in CM-CDCs was assessed by flow cytometry and shown in a representative figure. (B) Representative summary of the antigenic phenotype of CM-CDCs. (C) Representative summary of the antigenic phenotype of CLH-EDCs. Data are shown as the mean ± SEM of 3 independent experiments. *p < 0.05 vs. 0 h group, **p < 0.01 vs. 0 h group.
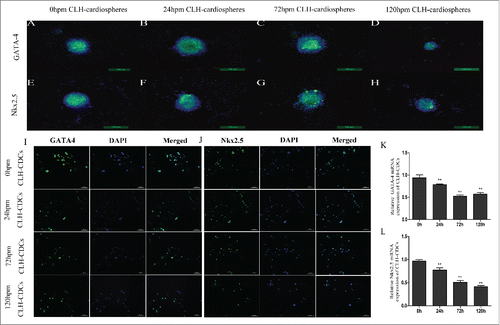
Transcription factors Nkx2.5 and GATA-4
Cadaver-like human cardiospheres (CLH-cardiospheres) post mortem expressed the cardiac-specific transcription factors GATA-4 and Nkx2.5 detected by immunohistochemistry (). CLH-EDCs also demonstrated widespread expression of GATA-4 and Nkx2.5 (). They expression in CLH-EDCs decreased gradually from 0 h to 120 h (p < 0.01; ). Similar findings were observed in CM-CDCs (Supplement ).
Figure 3. Comparison of transcription factors from human and mouse CDCs. Protein expression of GATA-4 and Nkx2.5 was measured by immunofluorescence and quantified by RT-PCR. (A-H) Human cardiospheres post mortem express GATA-4 and Nkx2.5 by immunofluorescence. (I and J) CLH-EDCs post mortem express GATA-4 and Nkx2.5 by immunofluorescence. Nuclei were counterstained with DAPI (blue) and cell positive in green. (K and L) CLH-EDCs post mortem express GATA-4 and Nkx2.5 by RT-PCR. Data are shown as the mean ± SEM of 3 independent experiments. (A-H. Scale bar = 100 µm, I-J. Scale bar = 50 µm) *p < 0.05 vs. 0 h group, **p < 0.01 vs. 0 h group.
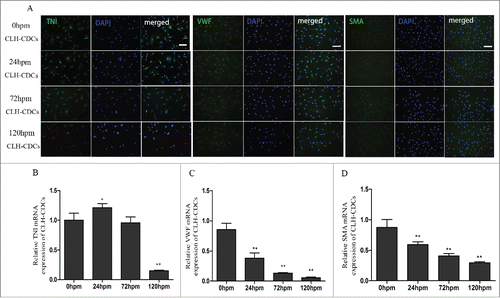
CDCs from human tissues have strong differentiation potential
Another potential advantage of CDCs is their reported differentiation potential. Their ability to undergo spontaneous cardiomyocyte, endothelial cell, and smooth muscle cell differentiation were examined in vitro. CLH-EDCs expressing TNI, VWF and SMA could be identified in every group. In CLH-EDCs, we found that TNI mRNA expression increased in the 24 h compared with 0 h group (p < 0.05; ). However, TNI levels were significantly increased in cadaveric mouse cardiomyocyte differentiation (Supplement ). With the extension of post mortem hours, the levels of VWF (p < 0.01, ) and SMA (p < 0.01, ) mRNA gradually decreased.
Figure 4. CLH-EDCs post mortem maintain their differentiation ability. We examined differentiation of CLH-EDCs post mortem by immunofluorescence and quantified by RT-PCR. (A) CLH-EDCs post mortem express TNI, VWF, and SMA by immunofluorescence. Nuclei were counterstained with DAPI (blue) and cell positive in green. (B-D) Quantitation of TNI, VWF, and SMA mRNA levels by RT-PCR. Scale bar = 100 µm. Data are shown as the mean ± SEM of 3 independent experiments. *p < 0.05 vs. 0 h group, **p < 0.01 vs. 0 h group.
In vitro secretion of growth factors
Increasing evidence supports the generalization that stem cell therapy boosts cardiac function largely via paracrine mechanisms. We thus compared the production of 3 growth factors (HGF, IGF-1, and VEGF) secreted by CLH-EDCs at different time points. There were no significant differences in productions of IGF-1 (), VEGF () and HGF () among 0 h, 24 h and 72 h. However, the productions of IGF-1 and VEGF were decreased in 120 h groups, while HGF did not.
Figure 5. CLH-EDCs post mortem have paracrine function. The comparison of cytokine profile secreted by CLH-EDCs. (A-C) Cytokines (VEGF, IGF-1, HGF) were analyzed by ELISA. Data are shown as the mean ± SEM of 3 independent experiments.*p < 0.05 vs. 0 h group.
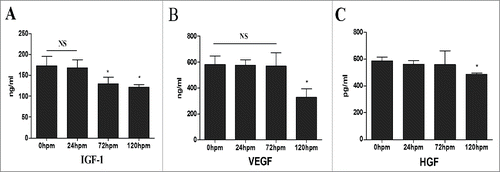
These data demonstrated that CLH-EDCs isolated 24 h post mortem retained paracrine function, which was a reason to improve cardiac function in vivo.
Changes in global cardiac function
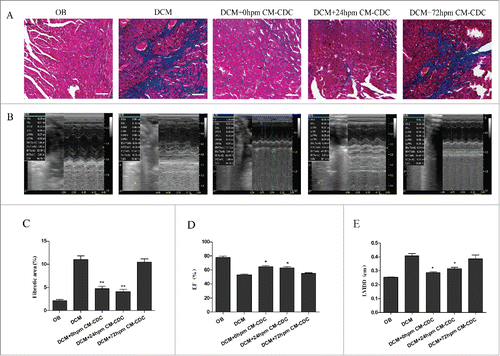
Cardiac function and myocardial fibrosis were assessed by echocardiography and Masson's trichrome staining. Myocardial fibrosis were evidently reduced in 0 h CM-CDCs-treated and 24 h CM-CDCs-treated groups, however fibrosis in the 72 h CM-CDCs-treated mice was similar to that of the PBS-treated group (). Eight weeks after transplantation of CM-CDCs, cardiac function was assessed by echocardiography in all groups (). Concomitantly, all echocardiographic data were seen in Supplement Table 2. We demonstrated that 24 h CM-CDCs-treated groups exhibited attenuated LV remodeling. Moreover, LVEF values increased in the 0 h (64.99 ± 3.4%) and 24 h CM-CDCs-treated groups (62.99 ± 2.8%) compared to the PBS-treated group (53.64 ± 5.6%); however, there was no statistical difference between the 0 h and 24 h CM-CDCs-treated groups (p = 0.51; ). Moreover, the LV internal diastolic diameter (LVIDD) decreased in the 0 h (0.29 ± 0.08 cm) and 24 h CM-CDCs-treated groups (0.32 ± 0.04 cm) compared to the PBS-treated group (0.41 ± 0.05 cm); there has no statistical difference between the 24 h and 0 h CM-CDCs-treated groups (p = 0.25; ).
Figure 6. CDCs transplantation reduce collagen mass and improve cardiac function. (A) Masson's trichrome staining for cardiac fibrosis evaluation. (B) Representative images of LV M-mode echocardiograms 8 weeks after CM-CDCs transplantation. (D and E) Changes in ejection fraction (EF) and LV internal diastolic diameter (LVIDD) after CM-CDCs or PBS treatment, n = 10 in PBS groups, n = 8 in CM-CDCs-treated groups. Scale bar = 50 µm. Data are shown as the mean ± SEM. *p < 0.05 vs. DCM+PBS treated group. (C) Myocardial fibrosis was evidently reduced in the 0 h CM-CDC-treated and 24 h CM-CDC-treated groups compared to the PBS-treated group; however, fibrosis in the 72 h CM-CDC-treated mice was similar to that in the PBS-treated mice.
Discussion
This is the first study to show that CDCs have a remarkable ability to survive for extended periods of time post mortem, in both humans and mice. We reported the isolation of viable CDCs from human biopsy specimens up to 120 h, and in mice up to 72 h post mortem. We found that TNI expression increased significantly in the 24 h group compared to the 0 h group. With the extension of post mortem hours, VWF and SMA mRNA levels gradually decreased. We also proved that human CDCs have similar paracrine function in the 24 h group compared to the 0 h group. Furthermore, we were the first to demonstrate that cadaveric CDCs can not only ameliorate myocardial fibrosis, but also improve cardiac function in a mouse model of DCM.
The use of cadaveric donors as a potential source of haematopoietic cells for transplantation purposesCitation15-16 has been shown in several publications for more than 50 y. Some studies suggested that human pluripotent haematopoietic stem cells could survive in cadaveric bone marrow(BM).Citation17-19 It has also been shown previously that split-thickness skin grafts could be allografted up to 3 weeks after death if stored at 4° C.Citation20 More recently, stem and adult cells have been isolated from post mortem brain, spinal cord, and retinal tissue.Citation3,21-23 These findings showed that, due to the persistence of stem cells, cadaver tissue could be a potential abundant source of these cells. We are the first to report that viable CDCs can be isolated post mortem, in both humans and mice.
It appears that many stem cells survive for hours or even days after death; however, no study thus far has systematically and quantitatively investigated the survival times of cadaveric cardiac stem cells. The isolation of viable and functional skeletal myogenic cells can be performed up to 17 d in humans, and up to 14 d in mice post mortem, much longer than previous reports recently described.Citation3 Erker found hepatic cells isolated up to 27 hours post mortem could be effectively used for liver cell therapy.Citation4 Moreover, Hjelm et al. demonstrated that cadaveric neuropheres were easier to obtain in early postnatal animals than in adult rats.Citation24 The isolation of viable and functional cadaveric stem cells from human bone marrow, brain, and muscle many days post mortem is possible today; but questions about cadaveric CDCs remained unsolved. In our study, we stored human cardiac tissue and mouse hearts at 4° C to simulate cadaveric conditions. We found that cadaveric CDCs from human biopsy specimens could be isolated up to 120 hours, and in mice up to 72 hours post mortem. CDCs obtained 24 h post mortem were not significantly different compared to those obtained at 0 h, with regards to viability and proliferation. GATA-4 and Nkx2.5 expression, as cardiac-specific transcription factors,Citation15 was decreased in the 24 h, 72 h, and 120 h groups compared to the 0 h group. In the current study, we further provided evidence that CDCs obtained 24 h post mortem could be a suitable source of donor cells.
Another potential advantage of CDCs is their reported ability to differentiate into cardiomyocytes, endothelial cells, and smooth muscle cells. Human cadaveric stem cells have also been reported to be capable of multilineage differentiation.Citation2,25 Post mortem human adipose tissue-derived stem cells were used to induce differentiation into myocardial-like cells.Citation26 A previous study showed that human cadaveric MSCs stored in liquid nitrogen for 5 y retained the ability to express VWF and CD31, supporting the commitment toward the endothelial cell lineage.Citation2 The above data suggests that human stem cells maintain their differentiation potential post mortem. In our study, we found that TNI expression even increased in the 24 h group compared to the 0 h group. Some suggest that severe hypoxia or anoxia is critical to maintaining stem cell viability and regenerative capacity, and may contribute to stem cell differentiation.Citation27-28 Based on the above results, we hypothesized that hypoxia may be helpful to induce myogenic differentiation.
CDCs secrete a variety of paracrine factors, such as IGF-1, HGF, VEGF, which have been shown to improve cardiac function.Citation29 Consistent with other findings, CDCs from heart failure patients secreted various growth factors, with no difference compared with non-heart failure CDCs.Citation29 Human CDCs maintained their ability to secrete large amounts of growth factors compared with BM mononuclear cells, BM-MSCs, adipose tissue-derived MSCs, and c-kit+ CDCs9. In our study, we found that human cadaveric CDCs could also secrete VEGF, HGF, and IGF-1. Importantly, VEGF and IGF-1 levels were no different between the 0 h and 24 h groups, but were decreased in the 120 h group (p < 0.05). Otherwise, there was no difference in HGF expression in any group. These data demonstrated that human CDCs isolated 24 h post mortem retained paracrine function, which was a reason to improve cardiac function in vivo.
Currently, cadaveric cells play an important role in regenerative medicine, which is gaining increasing attention. Cadaveric hepatocytes not only survived prolonged ischemia but also maintained their ability to engraft, repopulate, and correct metabolic liver disease in Fah−/− mice.Citation4 In another study, a human cadaveric corneal endothelial button could be used to treat more than one cornea of patients with diseased endothelium.Citation30 We found that intramyocardial injection of 24 h-CDCs post mortem could not only reduce cardiac collagen content, but also improve cardiac function in vivo. CDCs respond to oxidative stress by activating the Nrf2-Keap1 pathway; KLF5 expression leads to overproduction of collagen and exacerbates fibrosis, whose mechanisms have been proven in a transgenic mouse model of non-ischemic dilated cardiomyopathy.Citation13 However, these mechanisms require further confirmation in cadaveric CDCs in the future.
Conclusion
Viable CDCs can be isolated from human biopsy specimens up to 120 h, and in mice up to 72 h post mortem. Isolated CDCs are highly proliferative and multipotent, and can differentiate into different mesengenic lineages with paracrine function. In vivo, intramyocardial injection of CDCs obtained 24 h post mortem can also improve cardiac function. Based on these results, and with a useful and abundant stem cell number, the procurement of CDCs from cadaveric cardiac tissue 24 hour post mortem may be an alternative reservoir of CDCs for regenerative medicine and transplantation procedures.
Abbreviations
| BM | = | Bone marrow |
| CDCs | = | Cardiosphere-derived cells |
| CLH-EDCs | = | Cadaver-like human CDCs |
| CM-CDCs | = | Cadaver mouse CDCs |
| CM-EDCs | = | Cadaver mouse explant-derived cells |
| CLH-cardiospheres | = | Cadaver-like human cardiospheres |
| DAPI | = | 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride |
| DCM | = | Dilated cardiomyopathy |
| EDCs | = | Explant-derived cells |
| ELISA | = | Enzyme-linked immunoadsorbent assay |
| HGF | = | Hepatocyte growth factor |
| HE | = | Hematoxylin and eosin |
| IGF-1 | = | Insulin-like growth factor-1 |
| MSCs | = | Mesenchymal stem cells |
| MI | = | Myocardial infarction |
| SMA | = | Smooth muscle actin |
| TNI | = | Troponin I |
| VWF | = | von Willebrand factor |
| VEGF | = | Vascular endothelial growth factor |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Files
Download Zip (15.8 KB)Acknowledgments
We offer special thanks to International Science Editing for revision of the manuscript.
Funding
This work was supported by National Natural Science Foundation Projects to Dr. Jian Wu (81200240), the Postdoctoral Foundation of China (2013T60391), New Century Talents Foundation (1254-NCET-014), the Key Laboratory of Myocardial Ischemia, Chinese Ministry of Education, Harbin, Heilongjiang Province, China (KF201208, KF201405 and KF201406).
References
- Sousonis V, Nanas J, Terrovitis J. Cardiosphere-derived progenitor cells for myocardial repair following myocardial infarction. Curr Pharm Des 2014; 20:2003-11; PMID:23844734; http://dx.doi.org/10.2174/13816128113199990445
- Valente S, Alviano F, Ciavarella C, Buzzi M, Ricci F, Tazzari PL, Pagliaro P, Pasquinelli G. Human cadaver multipotent stromal/stem cells isolated from arteries stored in liquid nitrogen for 5 years. Stem Cell Res Ther 2014; 5:8-10; PMID:24429026; http://dx.doi.org/10.1186/scrt397
- Valente S, Alviano F, Ciavarella C, Buzzi M, Ricci F, Tazzari PL, Pagliaro P, Pasquinelli G. Skeletal muscle stem cells adopt a dormant cell state post mortem and retain regenerative capacity. Nat Commun 2012; 3:903; PMID:22692546; http://dx.doi.org/10.1038/ncomms1890
- Erker L, Azuma H, Lee AY, Guo C, Orloff S, Eaton L, Benedetti E, Jensen B, Finegold M, Willenbring H, et al. Therapeutic liver reconstitution with murine cells isolated long after death. Gastroenterology 2010; 139:1019-29; PMID:20621682; http://dx.doi.org/10.1053/j.gastro.2010.05.082
- Erker L, Azuma H, Lee AY, Guo C, Orloff S, Eaton L, Benedetti E, Jensen B, Finegold M, Willenbring H, Grompe M. et al. Isolation and expansion of adult cardiac stem cells from human and murine heart.Circ Res 2004; 95:911-21; PMID:15472116; http://dx.doi.org/10.1161/01.RES.0000147315.71699.51
- Kreke M, Smith RR, Marbán L, Marbán E. Cardiospheres and cardiosphere-derived cells as therapeutic agents following myocardial infarction. Expert Rev Cardiovasc Ther 2012; 10:1185-94; PMID:23098154; http://dx.doi.org/10.1586/erc.12.102
- Tang YL, Wang YJ, Chen LJ, Pan YH, Zhang L, Weintraub NL. Cardiac-derived stem cell-based therapy for heart failure: progress and clinical applications. Exp Biol Med (Maywood) 2013; 238:294-300; PMID:23598975; http://dx.doi.org/10.1177/1535370213477982
- Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 2009; 120:1075-83; PMID:19738142; http://dx.doi.org/10.1161/CIRCULATIONAHA.108.816058
- Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol 2012; 59:942-53; PMID:22381431; http://dx.doi.org/10.1016/j.jacc.2011.11.029
- Tseliou E, Pollan S, Malliaras K, Terrovitis J, Sun B, Galang G, Marbán L, Luthringer D, Marbán E. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. J Am Coll Cardiol 2013; 61:1108-19; PMID:23352785; http://dx.doi.org/10.1016/j.jacc.2012.10.052
- Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation 2012; 125:100-12; PMID:22086878; http://dx.doi.org/10.1161/CIRCULATIONAHA.111.042598
- Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 2012; 379:895-904; PMID:22336189; http://dx.doi.org/10.1016/S0140-6736(12)60195-0
- Aminzadeh MA, Tseliou E, Sun B, Cheng K, Malliaras K, Makkar RR, Marbán E. Therapeutic efficacy of cardiosphere-derived cells in a transgenic mouse model of non-ischaemic dilated cardiomyopathy. Eur Heart J 2014; 36:751-62; PMID:24866210; http://dx.doi.org/10.1093/eurheartj/ehu196
- Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007; 115:896-908; 17283259; http://dx.doi.org/10.1161/CIRCULATIONAHA.106.655209
- Mansilla E, Mártire K, Roque G, Tau JM, Marín GH, Castuma MV, Orlandi G, Tarditti A. Salvage of Cadaver Stem Cells (CSCs) as a Routine Procedure: History or Future for Regenerative Medicine. J Transplant Technol Res 2013; 3:118
- Marlicz W, Paczkowski M, Kijowski J, Machalinski B. Isolation of hematopoietic stem cells from heparinized cadaveric multiple organ donors: potential clinical implications. Transplant Proc 1999; 31:2099-2101; PMID:10455982; http://dx.doi.org/10.1016/S0041-1345(99)00275-4
- Michalova J, Savvulidi F, Sefc L, Forgacova K, Necas E. Cadaveric bone marrow as potential source of hematopoietic stem cells for transplantation. Chimerism 2011; 2:86-87; PMID:22163067; http://dx.doi.org/10.4161/chim.17917
- Raffoux C. Collaboration between hematopoietic stem cell donor registry and cord blood banks.Transplant Proc 2010; 42:3258-3259; PMID:20970667; http://dx.doi.org/10.1016/j.transproceed.2010.07.032
- Michalova J, Savvulidi F, Sefc L, Faltusova K, Forgacova K, Necas E. Hematopoietic stem cells survive circulation arrest and reconstitute hematopoiesis in myeloablated mice. Biol Blood Marrow Transplant 2011; 17:1273-81; PMID:21767513; http://dx.doi.org/10.1016/j.bbmt.2011.07.007
- Machaliński B, Paczkowski M, Kawa M, Paczkowska E, Ostrowski M. An optimization of isolation of early hematopoietic cells from heparinized cadaveric organ donors. Transplant Proc 2003; 35:3096-3100; PMID:14697988; http://dx.doi.org/10.1016/j.transproceed.2003.10.082
- Liu X, Zhu Y, Gao W. Isolation of neural stem cells from the spinal cords of low temperature preserved abortuses. J Neurosci Methods 2006; 157:64-70; PMID:16682082; http://dx.doi.org/10.1016/j.jneumeth.2006.03.025
- Donnenberg AD, Gorantla VS, Schneeberger S, Moore LR, Brandacher G, Stanczak HM, Koch EK, Lee WA. Clinical implementation of a procedure to prepare bone marrow cells from cadaveric vertebral bodies. Regen Med 2011; 6:701-706; PMID:22050522; http://dx.doi.org/10.2217/rme.11.89
- Gorantla VS, Schneeberger S, Moore LR, Donnenberg VS, Zimmerlin L, Lee WP, Donnenberg AD Development and validation of a procedure to isolate viable bone marrow cells from the vertebrae of cadaveric organ donors for composite organ grafting. Cytotherapy 2012; 14:104-113; PMID:21905958; http://dx.doi.org/10.3109/14653249.2011.605350
- Hjelm BE, Rosenberg JB, Szelinger S, Sue LI, Beach TG, Huentelman MJ, Craig DW. Induction of pluripotent stem cells from autopsy donor-derived somatic cells. Neurosci Lett 2011; 502:219-224; PMID:21839145; http://dx.doi.org/10.1016/j.neulet.2011.07.048
- Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2014; 2:606-19; PMID:24936449; http://dx.doi.org/10.1016/j.stemcr.2014.04.006
- Perán M, López-Ruiz E, González-Herrera L, Bustamante M, Valenzuela A, Marchal JA. Cellular extracts from post-mortem human cardiac tissue direct cardiomyogenic differentiation of human adipose tissue-derived stem cells. Cytotherapy 2013; 15:1541-8; http://dx.doi.org/10.1016/j.jcyt.2013.06.016
- Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res 2009; 104:1209-16; PMID:19407239; http://dx.doi.org/10.1161/CIRCRESAHA.109.197723
- Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, et al. Effect of stromal-cell-derived factor 1 on stemcell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 2003; 362:697-703; PMID:12957092; http://dx.doi.org/10.1016/S0140-6736(03)14232-8
- Cheng K, Malliaras K, Smith RR, Shen D, Sun B, Blusztajn A, Xie Y, Ibrahim A, Aminzadeh MA, Liu W, et al. Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair. JACC Heart Fail 2014; 2:49-61; PMID:24511463; http://dx.doi.org/10.1016/j.jchf.2013.08.008
- Xie Y, Ibrahim A, Cheng K, Wu Z, Liang W, Malliaras K, Sun B, Liu W, Shen D, Cheol Cho H, et al. Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells. Stem Cells 2014; 32:2397-406; PMID:24802280; http://dx.doi.org/10.1002/stem.1736
