ABSTRACT
G protein-coupled receptors (GPCRs) are critical players in tumor growth and progression. The redundant roles of GPCRs in tumor development confound effective treatment; therefore, targeting a single common signaling component downstream of these receptors may be efficacious. GPCRs transmit signals through heterotrimeric G proteins composed of Gα and Gβγ subunits. Hyperactive Gαs signaling can mediate tumor progression in some tissues; however, recent work in medulloblastoma and basal cell carcinoma revealed that Gαs can also function as a tumor suppressor in neoplasms derived from ectoderm cells including neural and epidermal stem/progenitor cells. In these stem-cell compartments, signaling through Gαs suppresses self-renewal by inhibiting the Sonic Hedgehog (SHH) and Hippo pathways. The loss of GNAS, which encodes Gαs, leads to activation of these pathways, over-proliferation of progenitor cells, and tumor formation. Gαs activates the cAMP-dependent protein kinase A (PKA) signaling pathway and inhibits activation of SHH effectors Smoothened-Gli. In addition, Gαs-cAMP-PKA activation negatively regulates the Hippo pathway by blocking the NF2-LATS1/2-Yap signaling. In this review, we will address the novel function of the signaling network regulated by Gαs in suppression of SHH-driven tumorigenesis and the therapeutic approaches that can be envisioned to harness this pathway to inhibit tumor growth and progression.
Introduction
The G protein coupled receptor (GPCR) signaling pathway plays critical roles in development, normal physiology, and disease. GPCRs transmit extracellular signals through heterotrimeric G proteins, which consist of three main subunits, Gα, Gβ, and Gγ. GDP-bound Gα is associated with Gβγ in an inactive state. Binding of ligands to GPCR causes exchange of GDP for GTP on Gα, leading to its dissociation from a membrane-anchored Gβγ complex. Downstream signaling from both Gα and Gβγ subunits is maintained until the GTPase activity of Gα hydrolyzes bound GTP to GDP, a process accelerated by Regulators of G protein Signaling (RGS) proteins.Citation1-3 G proteins have been linked to modulation of tumor growth, invasion, and metastasis, making this an important pathway for cancer therapy.Citation4-9 The Gα protein subunits differ in function and sequence homology. Gαs activates adenylyl cyclase and increases cytosolic cAMP levels; Gαi inhibits adenylyl cyclase and decreases intracellular cAMP levels; Gαq/11 activates phospholipase C; Gα12 and Gα13 regulate RhoA signaling via Rho-GEFs.Citation1-3 Heterotrimeric G proteins therefore represent a point of signaling convergence from multiple GPCRs, and they exert a pivotal role in mediating the functions of various GPCRs in cell growth and tumorigenesis.
Of these G protein subunits, Gαs, encoded by GNAS, is one of the most frequently mutated genes in cancer.Citation10 Many of these mutations in GNAS trigger gain-of-function of GPCR signaling that leads to enhanced intracellular cAMP levels, increased cell growth, and metastasis of human cancers.Citation1 In contrast, activating mutations in the opposing subunit, Gαi, decrease cAMP levels and are associated with adrenal cortical cancers and ovarian sex-cord tumors.Citation11 Thus, both elevation and reduction of cAMP levels may be oncogenic; it appears that an imbalance of intracellular cAMP may lead to an oncogenic transformation in a context-specific manner.Citation9 Recently, a series of genetic studies pointed to a critical role of GNAS in tumor suppression.Citation12-14 Genetic loss of a single GNAS allele in neural and skin progenitor cells causes medulloblastoma (MB) and basal cell carcinoma (BCC) respectively with full penetrance.Citation12,13 In this review, we will discuss the role of Gαs as a tumor suppressor by exploring underlying mechanisms whereby Gαs signaling regulates tumorigenesis through cAMP-dependent PKA, Sonic Hedgehog (SHH), and Hippo-LATS signaling pathways. We will further discuss how to target this novel tumor suppressive pathway for cancer treatment.
GNAS is a tumor suppressor gene in medulloblastoma
MBs are the most common malignant brain tumor in children, accounting for approximately 25% of all pediatric brain cancers. At present, molecular events and signaling pathways that drive the initiation and progression of these tumors are not fully understood. Mutations in genes encoding SHH signaling components Patched1, Smoothened (SMO), and Suppressor-of-fused (SUFU) account for approximately half of sporadic human SHH-subgroup MBs,Citation15,16 leading to hyperactivation of the SHH signaling pathway.
Analysis of two independent cohorts of SHH-associated MB patients in Boston and Heidelberg revealed that low expression of GNAS is correlated with significantly reduced overall survival.Citation12 Moreover, a recent report indicated that an infant carrying a homozygous nonsense mutation in GNAS developed aggressive MB.Citation17 These observations suggest that low expression or loss of GNAS specifically defines a subset of aggressive SHH-group MBs.
The loss of a single Gnas gene in neural progenitor cells is sufficient to initiate formation MB-like tumors in animal models.Citation12 The deletion of Gnas alleles in human glial fibrillary acidic protein (GFAP) promoter-expressing neural stem/progenitor cells, atonal homolog 1 (Atoh1) promoter-expressing cerebellar granular neuron progenitor cells (GNPs), or progenitors that express oligodendrocyte transcription factor 1 gene (Olig1) leads to an expansion of granule neuron progenitors and ultimately to formation of malignant SHH-associated MB in mice.Citation12 The tumors in these mice developed from anatomically distinct progenitors of the developing hindbrain recapitulating their human counterparts. Thus, Gnas is a critical determinant of progenitor cell competency and proliferation for MB initiation across disparate cells of origin. The identification of Olig1+ progenitor cells in the dorsal brainstem as the cellular origin for a subset of an anatomically distinct SHH-associated MB highlights the tumor heterogeneity with regard to cellular origin and anatomical location.
Gαs suppresses progenitor self-renewal and tumor formation in basal cell carcinoma
SHH signaling activation has been implicated in the etiology of the most common human cancer, basal cell carcinoma.Citation18 Mutations in the Patched gene, which negatively regulates SHH-SMO signaling have been identified in sporadic BCCs as well as those from patients with the rare genetic syndrome nevoid BCC.Citation18 When Gnas is knocked out in murine stem cells of the skin under an epidermal stem cell-specific promoter, the promoter that drives Keratin 14 expression, epidermal stem cells undergo uncontrolled proliferation, leading to the tumor lesions that resemble superficial and nodular human basal cell carcinoma.Citation13 Conversely, overexpression of Gαs in these same cells leads to premature differentiation of hair follicle stem cells and basal cells.Citation13 Thus, in both neural and skin progenitor populations, Gαs acts as a brake on excessive self-renewal or proliferation of progenitor cells.
GNAS methylation, which results in a low level of GNAS expression, has also been linked to poor prognosis in neuroblastoma.Citation19 Neuroblastoma is a neuroendocrine tumor, which arises from the neural crest cell lineage of the sympathetic nervous system. Thus, the tumor-suppressive action of Gαs is not limited to primordial neural progenitor cells in the cerebellum and hindbrain. Thus, current evidence suggests a broader role for Gαs in inhibiting multiple cancer types. One potential mechanism for the effect of GNAS loss in neural and epidermal progenitors is alteration in SHH and Hippo signaling pathways.
Gαs controls tumor formation by activating the PKA-cAMP signaling axis
Gαs suppresses SHH signal transduction through different cellular mechanisms. In the canonical signaling pathway, Gαs activation stimulates adenylyl cyclase activity to produce cAMP, which in turn activates the cAMP-dependent PKA. PKA is a major signaling effector of Gαs downstream of cAMP activation.Citation20,21 Activation of PKA has been shown to inhibit SHH signaling in a variety of cell types. PKA phosphorylates and inactivates Gli transcription factors, the SHH downstream effectors, and recruits the ubiquitin ligase β-TRCP. β-TRCP ubiquitinates Gli1 and Gli2, leading to their degradation, and enhances Gli3 processing into a Gli3R repressor form, thereby inhibiting SHH signaling.Citation22-24
The Gαs-cAMP-PKA signaling axis has an important role in suppression of MB and BCC tumors.Citation12,13 The loss of Gαs in the progenitor cells of the cerebellum and hindbrain leads to a decrease in intracellular cAMP levels and a reciprocal increase in SHH downstream target expression, leading to MB formation. Conversely, elevation of Gαs signaling effectors cAMP by either forskolin (an adenylyl cyclase agonist) or rolipram (a selective inhibitor of phosphodiesterase-4, PDE-4), which blocks cAMP degradation,Citation25,26 inhibits SHH signaling activation and reduces tumor cell proliferation and tumor size in the Gnas mutation-induced MB model.Citation12 Similarly, in basal stem cells of the skin, inhibition of PKA increases Gli-mediated transcription in vitro and leads to tumor formation, which phenocopies the tumorigenic phenotype in Gnas-mutant mice.Citation13 In addition, activation of cAMP-PKA via forskolin suppresses tumor growth in a K14-Rosa26-SmoM2 model of basal cell carcinoma.Citation27 Thus, there appears to be an inverse correlation between levels of cAMP/PKA activation and SHH signaling induced Gli-transcription-associated tumor growth. Because the loss of Gnas occurs independently of changes in other Hedgehog signaling components,Citation12 this Gαs-mediated signaling pathway may not only represent a novel mechanism for regulating the Hedgehog pathway but also underlie the drug resistance in MB treated with SMO antagonists alone.Citation28,29
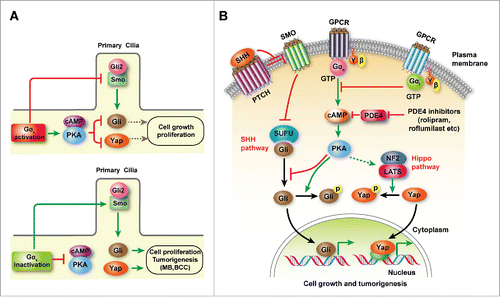
In addition to activation of PKA-cAMP intracellular events in murine cerebellar GNPs, Gαs activity also modulates SHH signaling component trafficking in the primary cilium, a structure believed to be a center for Hedgehog signaling.Citation30,31 Strikingly, Gαs protein is highly enriched at the primary cilium of GNPs.Citation12 Depletion of Gαs promotes the translocation of Gli2, a SHH downstream effector, onto the tip of primary cilia,Citation12 which activates the SHH signaling cascade. This is consistent with a role of PKA in restraining Gli2 activation.Citation32,33 Gαs can inhibit both ciliary translocation of SMO and Gli2 accumulation at the tip of primary cilia while maintaining the positioning of the SMO inhibitory protein Patched1 at the primary cilium.Citation12 This effect of Gαs on hedgehog signaling component trafficking provides an additional level of regulation of SHH signaling. Therefore, dual-mode regulation of both SHH signaling component trafficking at the primary cilia and cAMP-PKA mediated signaling cascade by Gαs activity reinforces the inhibition of SHH signaling activation and MB tumorigenesis ().
Gαs-PKA signaling suppresses Hippo signaling-mediated cell proliferation
In the Hippo pathway, signaling through kinases LATS1/2 and MST1/2 leads to phosphorylation and inactivation of the transcription factors Taz and Yap, the Hippo effectors that promote cell proliferation. Deletion of Gnas leads to an increase in Yap1 expression in BCC Citation13 and MB murine tumor models.Citation12 Consistently, in other mouse models of SHH-signaling induced MB, Yap1 is upregulated in cerebellar progenitor cells that express neural stem cell markers CD15 and nestin.Citation34
Inhibition of Yap1 expression in keratinocytes in the keratin 14-Cre-Gnas mouse BCC model leads to a profound reduction in colony formation.Citation13 This reduction appears to be even stronger than that caused by the loss of Gli1, pointing to a prominent role for the Hippo pathway in driving cell proliferation and self-renewal in tumors derived from epidermal progenitors.Citation13 Elevation of cAMP-PKA signaling induced by Gαs activity leads to LATS1 phosphorylation and activation. LATS1 in turn phosphorylates Yap1 to induce the cytoplasmic retention of Yap1 and thereby keep it in a transcriptionally inactive state. Furthermore, in a human keratinocyte cell line, inhibition of expression of LATS1/2 and NF2 (a co-regulator of LATS1/2) diminishes the cAMP-induced Yap1 phosphorylation,Citation13 suggesting that cAMP-dependent PKA can act on NF2/LATS1/2 to activate Hippo-LATS1/2 signaling to suppress Yap1 transcriptional activity ().
Loss of Gnas activates tumorigenic signaling and unmasks oncogenic activity of heterotrimeric G proteins
The coordinated and balanced activity of heterotrimeric G protein-mediated GPCR signaling regulates SHH and Hippo signaling to ensure proper tissue development and homeostasis by preventing uncontrolled cell growth.Citation13,14,35 The loss of Gαs may therefore disrupt the balance between pro-proliferative and pro-differentiation G proteins, leading to excessive signaling through pro-proliferative G proteins.
One of the potential oncogenic heterotrimeric G proteins that may regulate SHH signaling is Gαi. Gαi counteracts Gαs signaling by inhibiting production of intracellular cAMP. The GPCR-like SMO can interact with Gαi to activate Gli-dependent transcription in NIH 3T3 fibroblasts and in Drosophila.Citation36-38 Although the existence of SMO-Gαi coupling has been controversial,Citation39,40 it might represent a non-canonical branch of the pathway that activates Rac-RhoA-dependent signaling to enhance cell migration and proliferation.Citation41
Intriguingly, Gαi2 and Gαi3 are expressed in the external granular layer of rat cerebella and localized to the primary cilium. The loss of these heterotrimeric G proteins suppresses SHH-induced proliferation of cerebellar GNPs,Citation42 suggesting a potential role for Gαi signaling in MB formation. Recently, a cilia-enriched orphan GPCR Gpr175 (which has also been called Tpra1 or Tpra40) was shown to inhibit cAMP levels and activate SHH signaling through Gαi.Citation43 These findings are consistent with a model where Gαssuppresses, while Gαi promotes, oncogenic signaling in the primary cilium.
Recent studies indicate that Hippo signaling through Yap is suppressed by Gαs and is activated by a panoply of other heterotrimeric G proteins including Gα12/13, Gαq/11, Gα14, Gα15, and Gαi.Citation14,35,44 Of these, the most potent activators of Yap transcription are Gα12/13 and Gαq/11. Signaling through the lysophospholipid (LPA) receptor, a GPCR that couples to Gα12/13, drives serum-induced Yap transcription.Citation14 In addition, Gα12, Gα11, Gαq, and Gαi also regulate the activity of LATS1/2 kinases in Hek293T cells and uveal melanoma cells.Citation14,35,44 indicating a complex interplay between GPCRs and Hippo signaling. It is worth noting that EDG4, a member of the LPA receptor family, is overexpressed in Wnt and SHH subgroup MBs.Citation45 The functions of these heterotrimeric G proteins in MB formation remain to be defined. Nonetheless, the balance between GPCR signaling through adenylyl cyclase activator and suppressor heterotrimeric G proteins at least likely regulates tumorigenic events.
Loss of GNAS in neural and skin progenitors leads to tumor formation with full penetrance, suggesting a role of Gαs as a potent regulator of cell proliferation in SHH-signaling dependent progenitors originating from the neural tube and surface ectoderm during early lineage progression. In contrast, cancers in which hyperactivation of GNAS is oncogenic, such as in thyroid cancers and pituitary adenomas, arise from terminally differentiated cells derived from the endoderm.Citation1 This may suggest a potential correlation between cell type or stage in lineage progression and the effect of cAMP on tumorigenesis. Unraveling which cancers will respond positively, as opposed to negatively, to cAMP elevation is critical to the safe clinical application.
Therapeutic targeting of Gαs-cAMP-PKA signaling suppresses tumor growth
The studies of signaling events following dysregulation of heterotrimeric G proteins identified cAMP as a convergent downstream signaling node, making it an attractive target for tumor suppression. Several Gαs-coupled GPCRs that inhibit SHH target gene expression have been identified including GPR161 and PAC1.Citation46,47 These receptors activate PKA resulting in an increase in intracellular cAMP levels. Activation of the ciliary GPR161 elevates cAMP, leading to PKA activation and repression of Gli1/2 transcription.Citation46 At present, the role of GPR161 in tumor formation remains undefined. The PAC1 receptor, which binds the PACAP ligand also resulting in increased cAMP levels, has been shown to inhibit SHH signaling and Gli activation by PKA.Citation47 Reduced levels of PACAP enhance MB incidence in Patched heterozygous mice,Citation48 suggesting that signaling through PAC1 blocks the proliferation of GNPs during cerebellar development and MB formation.
Gαi-protein coupled receptors have been shown to synergize with Hedgehog signaling. CXCL12 stimulation of CXCR4, a Gαi coupled chemokine receptor, results in a significant reduction of intracellular cAMP levels and enhances the growth of SHH-driven medulloblastoma carrying an activated SmoA1 mutationCitation49,50 suggesting that CXCR4 activation maximizes proliferation of SHH-driven tumors. Inhibition of CXCR4 signaling via small molecule inhibitors AMD 3100 and AMD 3465 elevates cAMP levels and suppresses the growth of MB xenografts in vivo,Citation50 suggesting that dual inhibition of SHH and CXCR4 pathways may be beneficial for treating CXCR4-expressing SHH subtype MBs.
Phosphodiesterases, the enzymes responsible for the degradation of cAMP, have been shown to regulate MB growth.Citation51 Treatment with rolipram, an inhibitor of PDE4, suppresses SHH signaling and the growth of MB in Gnas-mutant mice without major changes in cerebellar architecture.Citation12 An unbiased in vivo chemical genetic screen identified that PDE4 inhibitors such as eggmanone exert a potent inhibitory effect on Hedgehog signaling.Citation52 PDE4 inhibition decreases the viability of the DAOY cell, an MB cell line.Citation53 In addition, blocking of PDE4D by roflumilast suppresses the growth of MB tumors resistant to the SHH antagonist vismodegib in mice,Citation54 whereas overexpression of PDE4A1, an isoform of PDE4, enhances the growth of DAOY cells in a mouse xenograft model.Citation55 Collectively, these studies suggest that PDE4, at least the A and D subtypes, represents a potential therapeutic target for SHH-dependent cancers. What is particularly exciting is that a number of PDE4 inhibitors such as rolipram and roflumilast have been used clinically for other indications and are well-tolerated while affording an avenue to tackle SHH antagonist resistance, raising hope in treating an otherwise challenging type of cancer ().
Concluding remarks
GPCR-Gαs signaling has long been considered an oncogenic pathway in human cancer; however, recent studies defined a novel tumor suppressive action of the Gαs protein in MB and BCC, suggesting that Gαs may function as a tumor suppressor in certain contexts. Future studies will determine whether GNAS plays a tumor-suppressive role in other primordial tumors of the developing nervous system such as pineoblastoma, supratentorial primitive neuroectodermal tumor, and neuroblastoma, the solid cancers most commonly observed in childhood. Targeting PDE4 with cAMP-raising agents has been shown to afford additional efficacy when combined with inhibitors of SMO to diminish the growth of MB cells Citation12 and to suppress the growth of vismodegib-resistant MB in mice.Citation54 This suggests that in combination with existing therapies, cAMP-raising agents might be repurposed to overcome multi-drug resistance in treatment of SHH-associated MBs.Citation56,57 Given that signaling control mediated via Gα proteins such as Gαs may be a point of signaling convergence for numerous GPCRs, targeting of Gαs and downstream pathway components such as cAMP-PKA may circumvent the drug resistance seen with SMO antagonists alone Citation28,29,58 and could be beneficial in the treatment of an array SHH-driven tumors including MB, BCC, small cell lung cancer, and pancreatic cancer.Citation22,29,59,60
Figure 1. Gαs-cAMP-PKA signaling suppresses progenitor proliferation and SHH-driven tumorigenesis. (A) A schematic diagram depicts the role of Gαs as a molecular switch that controls SHH-Gli and Hippo-Yap signaling activation. Gαs is highly enriched in the primary cilia of GNPs and blocks SMO-Gli ciliary translocation to block SMO activation. These Gαs-mediated intracellular cascades inhibit SHH-driven tumorigenic processes. Inactivation of Gαs activity in cerebellar and epidermal progenitors leads to activation of SHH-Gli and Hippo-Yap signaling and is sufficient to promote progenitor expansion and initiate MB and BCC formation, respectively. (B) GPCR-mediated Gαs activation, counterbalanced by Gαiactivity, increases cAMP levels and subsequently activates cAMP-dependent PKA signaling, leading to phosphorylation of Gli and Yap, the effectors of canonical SHH and Hippo signaling, respectively, and inactivation of their transcriptional activity for cell proliferation and tumorigenesis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to Drs. Xuelian He and Ed Hurlock for critical comments and Xianyao Zhou for assistance with the manuscript.
Funding
This study was funded in part by grants from the US National Institutes of Health (R01 NS078092 and R01 NS075243) to QRL.
References
- Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer 2007; 7:79-94; PMID:17251915; http://dx.doi.org/10.1038/nrc2069
- Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol 2009; 147:S46-S55; http://dx.doi.org/10.1038/sj.bjp.0706405
- Masuho I, Ostrovskaya O, Kramer GM, Jones CD, Xie K, Martemyanov KA. Distinct profiles of functional discrimination among G proteins determine the actions of G protein-coupled receptors. Sci Signal 2015; 8:ra123-ra; PMID:26628681; http://dx.doi.org/10.1126/scisignal.aab4068
- Yoda A, Adelmant G, Tamburini J, Chapuy B, Shindoh N, Yoda Y, Weigert O, Kopp N, Wu SC, Kim SS, et al. Mutations in G protein β subunits promote transformation and kinase inhibitor resistance. Nat Med 2015; 21:71-5; PMID:25485910; http://dx.doi.org/10.1038/nm.3751
- Ward JD, Ha JH, Jayaraman M, Dhanasekaran DN. LPA-mediated migration of ovarian cancer cells involves translocalization of Gαi2 to invadopodia and association with Src and β-pix. Cancer Letters 2015; 356:382-91; PMID:25451317; http://dx.doi.org/10.1016/j.canlet.2014.09.030
- Wang Z, Dela Cruz R, Ji F, Guo S, Zhang J, Wang Y, Feng GS, Birnbaumer L, Jiang M, Chu WM. G(i)α proteins exhibit functional differences in the activation of ERK1/2, Akt and mTORC1 by growth factors in normal and breast cancer cells. Cell Communication Signal 2014; 12:10; http://dx.doi.org/10.1186/1478-811X-12-10
- Choi YJ, Kim SY, Oh JM, Juhnn YS. Stimulatory heterotrimeric G protein augments gamma ray-induced apoptosis by up-regulation of Bak expression via CREB and AP-1 in H1299 human lung cancer cells. Exp Mol Med 2009; 41:592-600; PMID:19381065; http://dx.doi.org/10.3858/emm.2009.41.8.065
- Kumar KK, Burgess AW, Gulbis JM. Structure and function of LGR5: an enigmatic G-protein coupled receptor marking stem cells. Protein Sci 2014; 23:551-65; PMID:24677446; http://dx.doi.org/10.1002/pro.2446
- O'Hayre M, Degese MS, Gutkind JS. Novel insights into G protein and G protein-coupled receptor signaling in cancer. Curr Opin Cell Biol 2014; 27:126-35; PMID:24508914; http://dx.doi.org/10.1016/j.ceb.2014.01.005
- Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 2010; 466:869-73; PMID:20668451; http://dx.doi.org/10.1038/nature09208
- O'Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, Gutkind JS. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer 2013; 13:412-24; PMID:23640210; http://dx.doi.org/10.1038/nrc3521
- He X, Zhang L, Chen Y, Remke M, Shih D, Lu F, Wang H, Deng Y, Yu Y, Xia Y, et al. The G protein α subunit Galphas is a tumor suppressor in Sonic hedgehog-driven medulloblastoma. Nat Med 2014; 20:1035-42; PMID:25150496; http://dx.doi.org/10.1038/nm.3666
- Iglesias-Bartolome R, Torres D, Marone R, Feng X, Martin D, Simaan M, Chen M, Weinstein LS, Taylor SS, Molinolo AA, et al. Inactivation of a Gαs-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol 2015; 17(6):793-803:advance on; PMID:25961504; http://dx.doi.org/10.1038/ncb3164
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012; 150:780-91; PMID:22863277; http://dx.doi.org/10.1016/j.cell.2012.06.037
- Kool M, Jones DT, Jager N, Northcott PA, Pugh TJ, Hovestadt V, Piro RM, Esparza LA, Markant SL, Remke M, et al. Genome Sequencing of SHH Medulloblastoma Predicts Genotype-Related Response to Smoothened Inhibition. Cancer Cell 2014; 25:393-405; PMID:24651015; http://dx.doi.org/10.1016/j.ccr.2014.02.004
- Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 2012; 123:465-72; PMID:22134537; http://dx.doi.org/10.1007/s00401-011-0922-z
- Huh JY, Kwon MJ, Seo KY, Kim MK, Chae KY, Kim SH, Ki CS, Yoon MS, Kim DH. Novel nonsense GNAS mutation in a 14-month-old boy with plate-like osteoma cutis and medulloblastoma. J Dermatol 2014; 41(4):319-21; PMID:24517547; http://dx.doi.org/10.1111/1346-8138.12284
- Lupi O. Correlations between the Sonic Hedgehog pathway and basal cell carcinoma. Int J Dermatol 2007; 46:1113-7; PMID:17988327; http://dx.doi.org/10.1111/j.1365-4632.2007.03391.x
- Decock A, Ongenaert M, Hoebeeck J, De Preter K, Van Peer G, Van Criekinge W, Ladenstein R, Schulte JH, Noguera R, Stallings RL, et al. Genome-wide promoter methylation analysis in neuroblastoma identifies prognostic methylation biomarkers. Genome Biol 2012; 13:R95-R; PMID:23034519; http://dx.doi.org/10.1186/gb-2012-13-10-r95
- Taylor SS, Zhang P, Steichen JM, Keshwani MM, Kornev AP. PKA: lessons learned after twenty years. Biochim Biophys Acta 2013; 1834:1271-8; PMID:23535202; http://dx.doi.org/10.1016/j.bbapap.2013.03.007
- Stratakis CA. cAMP/PKA signaling defects in tumors: genetics and tissue-specific pluripotential cell-derived lesions in human and mouse. Mol Cell Endocrinol 2013; 371:208-20; PMID:23485729; http://dx.doi.org/10.1016/j.mce.2013.01.015
- Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med 2013; 19:1410-22; PMID:24202394; http://dx.doi.org/10.1038/nm.3389
- Chen Y, Jiang J. Decoding the phosphorylation code in Hedgehog signal transduction. Cell Res 2013; 23:186-200; PMID:23337587; http://dx.doi.org/10.1038/cr.2013.10
- Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol 2007; 17:438-47; PMID:17845852; http://dx.doi.org/10.1016/j.tcb.2007.06.007
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A 2004; 101:8786-90; PMID:15173585; http://dx.doi.org/10.1073/pnas.0402595101
- Conti M, Jin SL. The molecular biology of cyclic nucleotide phosphodiesterases. Prog Nucleic Acid Res Mol Biol 1999; 63:1-38; PMID:10506827; http://dx.doi.org/10.1016/S0079-6603(08)60718-7
- Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 2012; 337:1357-60; PMID:22984073; http://dx.doi.org/10.1126/science.1220845
- Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science 2009; 326:572-4; PMID:19726788; http://dx.doi.org/10.1126/science.1179386
- Ng JM, Curran T. The Hedgehog's tale: developing strategies for targeting cancer. Nat Rev Cancer 2011; 11:493-501; PMID:21614026; http://dx.doi.org/10.1038/nrc3079
- Nozawa YI, Lin C, Chuang PT. Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Curr Opin Genet Dev 2013; 23(4):429-37; PMID:23725801; http://dx.doi.org/10.1016/j.gde.2013.04.008
- Goetz SC, Ocbina PJ, Anderson KV. The primary cilium as a Hedgehog signal transduction machine. Methods Cell Biol 2009; 94:199-222; PMID:20362092; http://dx.doi.org/10.1016/S0091-679X(08)94010-3
- Pan Y, Wang C, Wang B. Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development. Dev Biol 2009; 326:177-89; PMID:19056373; http://dx.doi.org/10.1016/j.ydbio.2008.11.009
- Tuson M, He M, Anderson KV. Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube. Development 2011; 138:4921-30; PMID:22007132; http://dx.doi.org/10.1242/dev.070805
- Fernandez-L A, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, Taylor MD, Kenney AM. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev 2009; 23:2729-41; PMID:19952108; http://dx.doi.org/10.1101/gad.1824509
- Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell 2014; 25:822-30; PMID:24882516; http://dx.doi.org/10.1016/j.ccr.2014.04.017
- Riobo NA, Saucy B, Dilizio C, Manning DR. Activation of heterotrimeric G proteins by Smoothened. Proc Natl Acad Sci U S A 2006; 103:12607-12; PMID:16885213; http://dx.doi.org/10.1073/pnas.0600880103
- Ogden SK, Fei DL, Schilling NS, Ahmed YF, Hwa J, Robbins DJ. G protein Galphai functions immediately downstream of Smoothened in Hedgehog signalling. Nature 2008; 456:967-70; PMID:18987629; http://dx.doi.org/10.1038/nature07459
- Carbe CJ, Cheng L, Addya S, Gold JI, Gao E, Koch WJ, Riobo NA. Gi proteins mediate activation of the canonical hedgehog pathway in the myocardium. AJP: Heart Circulatory Physiol 2014; 307:H66-H72
- Low WC, Wang C, Pan Y, Huang XY, Chen JK, Wang B. The decoupling of Smoothened from Galphai proteins has little effect on Gli3 protein processing and Hedgehog-regulated chick neural tube patterning. Dev Biol 2008; 321:188-96; PMID:18590719; http://dx.doi.org/10.1016/j.ydbio.2008.06.014
- Philipp M, Caron MG. Hedgehog signaling: is Smo a G protein-coupled receptor? Curr Biol 2009; 19:R125-7; PMID:19211052; http://dx.doi.org/10.1016/j.cub.2008.12.010
- Polizio AH, Chinchilla P, Chen X, Kim S, Manning DR, Riobo NA. Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J Biol Chem 2011; 286:19589-96; PMID:21474452; http://dx.doi.org/10.1074/jbc.M110.197111
- Barzi M, Kostrz D, Menendez A, Pons S. Sonic Hedgehog-induced proliferation requires specific Gα inhibitory proteins. J Biol Chem 2011; 286:8067-74; PMID:21209076; http://dx.doi.org/10.1074/jbc.M110.178772
- Singh J, Wen X, Scales SJ. The Orphan G Protein-Coupled Receptor Gpr175 (TPRA40) Enhances Hedgehog Signaling by Modulating cAMP Levels. J Biol Chem 2015:M115.665810-M115
- Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, et al. Hippo-Independent Activation of YAP by the GNAQ Uveal Melanoma Oncogene through a Trio-Regulated Rho GTPase Signaling Circuitry. Cancer Cell 2014; 25:831-45; PMID:24882515; http://dx.doi.org/10.1016/j.ccr.2014.04.016
- Whittier KL, Boese EA, Gibson-Corley KN, Kirby PA, Darbro BW, Qian Q, Ingram WJ, Robertson T, Remke M, Taylor MD, et al. G-protein coupled receptor expression patterns delineate medulloblastoma subgroups. Acta Neuropathologica Communications 2013; 1:66; PMID:24252460; http://dx.doi.org/10.1186/2051-5960-1-66
- Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell 2013; 152:210-23; PMID:23332756; http://dx.doi.org/10.1016/j.cell.2012.12.026
- Niewiadomski P, Zhujiang A, Youssef M, Waschek JA. Interaction of PACAP with Sonic hedgehog reveals complex regulation of the hedgehog pathway by PKA. Cell Signal 2013; 25:2222-30; PMID:23872071; http://dx.doi.org/10.1016/j.cellsig.2013.07.012
- Lelievre V, Seksenyan A, Nobuta H, Yong WH, Chhith S, Niewiadomski P, Cohen JR, Dong H, Flores A, Liau LM, et al. Disruption of the PACAP gene promotes medulloblastoma in ptc1 mutant mice. Dev Biol 2008; 313:359-70; PMID:18036580; http://dx.doi.org/10.1016/j.ydbio.2007.10.031
- Yang L, Jackson E, Woerner BM, Perry A, Piwnica-Worms D, Rubin JB. Blocking CXCR4-mediated cyclic AMP suppression inhibits brain tumor growth in vivo. Cancer Res 2007; 67:651-8; PMID:17234775; http://dx.doi.org/10.1158/0008-5472.CAN-06-2762
- Sengupta R, Dubuc A, Ward S, Yang L, Northcott P, Woerner BM, Kroll K, Luo J, Taylor MD, Wechsler-Reya RJ, et al. CXCR4 activation defines a new subgroup of Sonic hedgehog-driven medulloblastoma. Cancer Res 2012; 72:122-32; PMID:22052462; http://dx.doi.org/10.1158/0008-5472.CAN-11-1701
- Sengupta R, Sun T, Warrington NM, Rubin JB. Treating brain tumors with PDE4 inhibitors. Trends Pharmacol Sci 2011; 32:337-44; PMID:21450351; http://dx.doi.org/10.1016/j.tips.2011.02.015
- Williams CH, Hempel JE, Hao J, Frist AY, Williams MM, Fleming JT, Sulikowski GA, Cooper MK, Chiang C, Hong CC. An In Vivo Chemical Genetic Screen Identifies Phosphodiesterase 4 as a Pharmacological Target for Hedgehog Signaling Inhibition. Cell Reports 2015; 11:43-50; PMID:25818300; http://dx.doi.org/10.1016/j.celrep.2015.03.001
- Schmidt AL, de Farias CB, Abujamra AL, Kapczinski F, Schwartsmann G, Brunetto AL, Roesler R. BDNF and PDE4, but not the GRPR, regulate viability of human medulloblastoma cells. J Mol Neurosci 2010; 40:303-10; PMID:19642024; http://dx.doi.org/10.1007/s12031-009-9221-8
- Ge X, Milenkovic L, Suyama K, Hartl T, Purzner T, Winans A, Meyer T, Scott MP. Phosphodiesterase 4D acts downstream of Neuropilin to control Hedgehog signal transduction and the growth of medulloblastoma. Elife 2015; 4:e07068; http://dx.doi.org/10.7554/eLife.07068
- Goldhoff P, Warrington NM, Limbrick DD, Jr, Hope A, Woerner BM, Jackson E, Perry A, Piwnica-Worms D, Rubin JB. Targeted inhibition of cyclic AMP phosphodiesterase-4 promotes brain tumor regression. Clin Cancer Res 2008; 14:7717-25; PMID:19047098; http://dx.doi.org/10.1158/1078-0432.CCR-08-0827
- Chahar MK, Sharma N, Dobhal MP, Joshi YC. Flavonoids: A versatile source of anticancer drugs. Pharmacogn Rev 2011; 5:1-12; PMID:22096313; http://dx.doi.org/10.4103/0973-7847.79093
- Nikaido T, Ohmoto T, Kinoshita T, Sankawa U, Delle Monache F, Botta B, Tomimori T, Miyaichi Y, Shirataki Y, Yokoe I, et al. Inhibition of adenosine 3',5'-cyclic monophosphate phosphodiesterase by flavonoids. III. Chem Pharm Bull (Tokyo) 1989; 37:1392-5; PMID:2560949; http://dx.doi.org/10.1248/cpb.37.1392
- Rodon J, Tawbi HA, Thomas AL, Stoller RG, Turtschi CP, Baselga J, Sarantopoulos J, Mahalingam D, Shou Y, Moles MA, et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res 2014; 20:1900-9; PMID:24523439; http://dx.doi.org/10.1158/1078-0432.CCR-13-1710
- Kar S, Deb M, Sengupta D, Shilpi A, Bhutia SK, Patra SK. Intricacies of hedgehog signaling pathways: a perspective in tumorigenesis. Exp Cell Res 2012; 318:1959-72; PMID:22659135; http://dx.doi.org/10.1016/j.yexcr.2012.05.015
- Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta 2010; 1805:181-208; PMID:20085802
