ABSTRACT
The mammalian target of rapamycin (mTOR) plays essential roles in the regulation of growth-related processes such as protein synthesis, cell sizing and metabolism in both normal and pathological growing conditions. These functions of mTOR are thought to be largely a consequence of its cytoplasmic activity in regulating translation rate, but accumulating data highlight supplementary role(s) for this serine/threonine kinase within the nucleus. Indeed, the nuclear activities of mTOR are currently associated with the control of protein biosynthetic capacity through its ability to regulate the expression of gene products involved in the control of ribosomal biogenesis and proliferation. Using primary murine embryo fibroblasts (MEFs), we observed that cells with overactive mTOR signaling displayed higher abundance for the growth-associated Npm1 protein, in what represents a novel mechanism of Npm1 gene regulation. We show that Npm1 gene expression is dependent on mTOR as demonstrated by treatment of wild-type and Pten inactivated MEFs cultured with rapamycin or by transient transfections of small interfering RNA directed against mTOR. In accordance, the mTOR kinase localizes to the Npm1 promoter gene in vivo and it enhances the activity of a human NPM1-luciferase reporter gene providing an opportunity for direct control. Interestingly, rapamycin did not dislodge mTOR from the Npm1 promoter but rather strongly destabilized the Npm1 transcript by increasing its turnover. Using a prostate-specific Pten-deleted mouse model of cancer, Npm1 mRNA levels were found up-regulated and sensitive to rapamycin. Finally, we also showed that Npm1 is required to promote mTOR-dependent cell proliferation. We therefore proposed a model whereby mTOR is closely involved in the transcriptional and posttranscriptional regulation of Npm1 gene expression with implications in development and diseases including cancer.
Introduction
The mammalian target of rapamycin (mTOR) is a highly conserved serine/threonine protein kinase that regulates cell growth and metabolism in response to nutrient availability, growth factor and cellular stresses.Citation1,2 It belongs to the family of phosphatidylinositol 3-kinase (PI3K)-related kinases (PIKKs) and operates in two functionally distinct cellular complexes termed mTOR complex 1 (mTORC1) and 2 (mTORC2) which associate respectively the regulatory-associated protein of mTOR (raptor) and the rapamycin-insensitive companion of mTOR (rictor).Citation3,4 Once activated, mTORC1 promotes cell growth through enhanced translation resulting from activation of the ribosomal S6 subunit kinase (S6K) and inactivation of the eukaryotic translation initiation factor 4E (eIF4E)-binding protein (4E-BP1).Citation5 This growth-promoting activity which is further strengthened by mTORC2-dependent functions is now also described as able to activate a transcriptional program that is critical for protein synthesis and cell growth. Therefore, through its binding to the promoters of RNA polymerase I and III transcribed genes, mTOR increases the abundance of rRNAs (rRNA) and tRNAs (tRNA) to enhance protein biosynthetic capacity and increase cell growth.Citation6
Recent studies have identified additional gene regulatory networks downstream of mTORC1 complex that might activate specific cellular processes that are likely to contribute to human physiology and disease. Using a genome-wide scale analysis, Chaveroux et al.Citation7 demonstrated that mTOR occupies many regulatory regions on chromatin obtained from mouse liver with a strong enrichment at the proximal promoter of the Npm1 gene. This particular gene encodes the abundant and ubiquitously expressed nucleophosmin protein (Npm1 also known as B23, numatrin and NO38) which has been originally identified as a non-ribosomal nucleolar phosphoprotein found at high levels in the granular regions of the nucleus.Citation8 It is largely accumulated in proliferating and embryonic stem cells compared to non-dividing resting cells,Citation9,10 and its accumulation is markedly and promptly increased in association with mitogensCitation11,12 or some cellular stresses.Citation13-15 As for mTOR, Npm1 expression is altered in several different types of cancer and is demonstrated to be a positive regulator of ribosome biogenesis, cell proliferation and resistance to cell death. Citation16 To date, the regulatory mechanisms involved in the regulation of the Npm1 gene expression are not fully understood but experimental evidence demonstrated that transcription factors such as YY1 and Myc can both bind directly to the 5′ region of the Npm1 gene.Citation17,18 In addition to the activity of the mTOR kinase to control mitochondrial oxidative activities and metabolism,Citation19,20 its recruitment to the Npm1 promoter region prompted us to address whether mTOR targets the Npm1 gene to modulate its expression at a transcriptional level. A vast amount of tumors display aberrant mTOR activity including prostate tumors for which high level of Npm1 is proposed to enhance tumor cells aggressiveness.Citation21,22 However, it is currently unknown to which extent these two key regulators of cell proliferation might interact to regulate common biological functions with implications in diseases and cancers.
By using mouse embryonic fibroblasts (MEFs) inactivated for the tumor suppressor gene Pten, (phosphatase and tensin homolog deleted on chromosome 10), a negative regulator of the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR signaling pathway, we demonstrate that the mRNA and the protein levels of Npm1 are increased in Pten deficient MEFs with hyperactive mTOR signaling when compared to with wild-type fibroblasts. Furthermore, we show that both rapamycin inhibition and RNA silencing of mTOR reduce Npm1 expression indicating that mTOR is involved in this control. By using a chromatin immunoprecipitation (ChIP) assay in conjunction with a reporter luciferase assay, we find that mTOR binds to the promoter of the Npm1 target gene to regulate its expression. Finally, we find that Npm1 levels are increased in prostate tumors of mutant mice with genetic inactivation of Pten, a bona fide animal model that has been described to recapitulate all spectrums of human prostate cancer, from tumor initiation to metastasis.Citation23 When reproduced in MEFs, Pten deletion induces mTOR activation, upregulated Npm1 expression and increased proliferation that is dependent both of mTOR and Npm1. Taken together; these results raised the possibility that part of the deleterious activity of mTOR in cancer cells is dependent on its ability to induce Npm1 expression. In that regard, our data may help to clarify the mechanisms underlying the transcriptional regulation of the Npm1 gene and may shed light on the ability of mTOR to control Npm1 functions in particular regarding the effect of mTOR in cell proliferation and neoplastic transformation.
Results
Pten-null fibroblasts display higher expression levels of Npm1
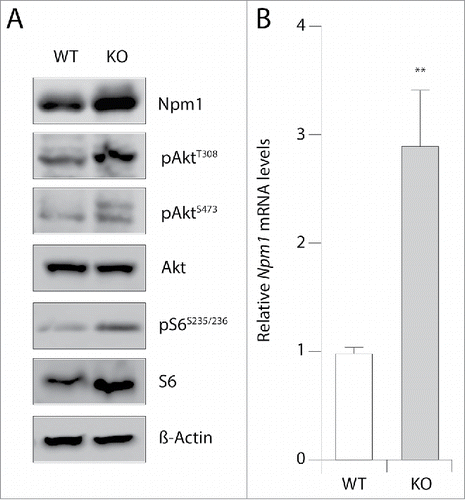
Despite the multitude functions of Npm1 in a plethora of biological processes, the molecular mechanisms of Npm1 gene activation are still poorly understood. Recent data emerging from genome-wide chromatin immunoprecipitation analyses indicate that the highly conserved mTOR protein kinase localized at many regions surrounding the transcriptional starting site of genes with a significant enrichment at the Npm1 promoter gene.Citation7 This suggests that mTOR may regulate Npm1 abundance. To address the relationship between these two proteins, we used a mouse embryonic fibroblast (MEF) cell line with constitutive Pten inactivation. These cells display increased Akt Thr-308 phosphorylation levels due to overactive phosphatidylinositol 3-kinase (PI3K) signaling pathway. In turn, this increases phosphorylation levels of the downstream S6 ribosomal (Ser235/236) target protein and enhances Akt Ser-473 phosphorylation respectively by mTORC1 and mTORC2 as already described.Citation24 Immunoblot analyses of cell lysates show that Pten-null mouse embryo fibroblasts have increased levels of Npm1 protein compared with their wild-type counterparts (). This change in protein abundance was correlated with a 2.5- to 3.0-fold increase in Npm1 mRNA levels after normalization to the house keeping gene 36b4 (p < 0.01) by qRT-PCR assays (). These data indicate that enhanced activation of mTOR due to sustained PI3K/Akt signaling in MEFs lacking Pten may contribute to regulate positively Npm1 expression at a transcriptional and/or posttranscriptional level.
Figure 1. Npm1 mRNA and protein levels are enhanced in MEF cells inactivated for Pten. (A) Thirty µg of total protein extracts from wild-type (WT) and Pten knockout (KO) mouse embryonic fibroblasts were separated by SDS-PAGE and immunoblotted with specific antibodies as indicated (n = 3). (B) RT-qPCR analysis of Npm1 mRNA accumulation in WT and Pten KO MEFs (normalized to 1.0 relative to 36b4 mRNA gene for control WT MEFs). Bar graphs show the mean level of three independent experiments (±SEM) and a student's test was performed to assess statistical significance. **p < 0.01.
Npm1 expression is affected by mTOR inhibition
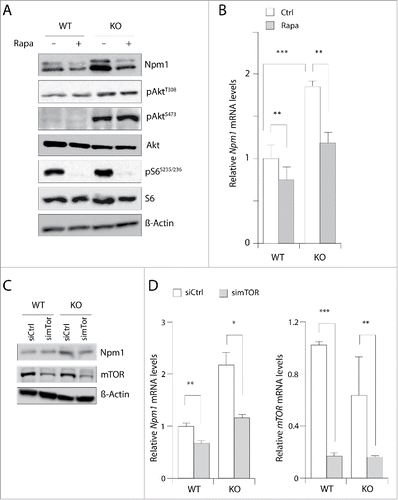
In an attempt to define if mTOR was responsible for nucleophosmin accumulation, we treated wild-type and Pten negative MEF cultures with 20 nM of the allosteric mTOR inhibitor rapamycin for 24 hours and compared the amounts of Npm1 by western blotting. This long-term treatment should likely inhibit both mTOR complexes since previous studies have shown that rapamycin binds to the intracellular protein FKBP12 to generate a drug-receptor complex that then tethers a large fraction of the free mTOR molecules to inhibit the kinase activity of mTORC1 and suppress the assembly and function of mTORC2.Citation25 Unphosphorylated S6 was used as a loading control and phosphos-S6 (Ser235/236) showed that mTORC1 signaling, which stimulates phosphorylation of the ribosomal S6 protein at this site, was inhibited by rapamycin. The experiment in showed that addition of rapamycin compared to vehicle only slightly reduced Npm1 protein levels in wild-type MEFs but reduced by 50% (p = 0.09) the protein levels in Pten-deleted MEFs cells, in which mTOR is over activated and Npm1 overexpressed. This fall in Npm1 abundance unlikely results from a reduction in global translation since the S6 protein levels are unaffected in the same condition. Contrary to a recent report,Citation26 the fall we observed in Npm1 abundance is likely to occur in part at the transcriptional level since Npm1 mRNA levels are reduced by 25% (p < 0.01) and 40% (p < 0.01) respectively in wild-type and Pten-null MEFs after 4 h of treatment with 20 nM rapamycin (). As shown in , prolonged treatment with rapamycin did not lead to the loss of phospho-Akt (S473) in MEF with Pten loss (compare lanes 3 and 4) indicating that the assembly of mTORC2 is unlikely blocked and that kinases upstream of mTOR (i.e. Akt) may not be completely inhibited in these experimental conditions. To circumvent this later point, higher doses of rapamycin were used to efficiently reduce both Akt Thr-308 and Ser-473 phosphorylation levels. However Npm1 abundance is not decreased further in these conditions indicating thus that mTORC2 complex is unlikely involved in Npm1 down-regulation (supplemental ). Nevertheless, these data clearly pointed to a role of mTOR and, to confirm this possibility, we decided to knockdown mTOR in Pten-deficient MEFs. This triggered a similar decrease in Npm1 mRNA levels and protein abundance, as mTOR mRNA abundance is itself decreased by more than 80% (). Together, our data are consistent with a transcriptional and/or posttranscriptional regulation of the Npm1 gene by mTOR complexes.
Figure 2. Inhibition of mTOR signaling reduces Npm1 expression in MEFs. (A) Cultured wild-type (WT) and Pten knockout (KO) cells were treated for 24 hours in the absence or the presence of 20 nM rapamycin. Cell extracts were prepared and aliquots containing 30 µg of total proteins were resolved by SDS-PAGE for proteins visualization by Western blotting. (B) Cells were incubated as above except that rapamycin was added to the medium for 4 hours. Npm1 and mTOR expression levels were then assayed by RT-qPCR from mRNA isolated from WT and KO MEFs for Pten and levels were normalized by comparison to the 36b4 mRNA. Bar graphs show the mean levels of at least three independent experiments (±SEM) of qPCR-amplified Npm1 (normalized to 1.0 for control untreated WT MEF cells). (C) Western blot analysis of total Npm1 and mTOR in MEF cells treated with a pool of siRNA (50 nM) for 48 hours against mTOR. β-Actin levels are shown as a loading control. (D) RT-qPCR analysis of Npm1 expression after depletion of mTOR. Errors bars represent mean ± SEM normalized to WT untreated cells (n = 3; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
mTOR binds to the Npm1 promoter and modulates its transcriptional activity
To confirm the above observations, we examined the DNA binding activity of mTOR in MEF cultures using standard chromatin immunoprecipitation (ChIP). As shown in , mTOR immunoprecipitated DNA was analyzed with PCR primer pairs spanning the Npm1 promoter region and the pol III-transcribed tRNAArg/Tyr positive control gene (see Materials and Methods). In lines with previous reports, mTOR specifically targets the pol-III transcribed tRNAArg/Tyr gene, but not a non-mTOR-bound genomic region (negative control) thus confirming the specificity of the reaction (). Examination of multiple experiments confirmed the association of endogenous mTOR to the upstream region of the Npm1 gene in wild-type MEFs cultured in normal growth conditions (). This region encompasses the peak found by Chaveroux et al. at −425 from the transcription starting site and seems to be specific of the ChIP assay since no signal was detected with control IgG. Interestingly, Pten loss did not substantially increased mTOR occupancy since we detected equivalent amount of mTOR associated at this promoter (). Moreover, treatment with rapamycin did not reduce the level of mTOR occupancy at the Npm1 promoter region suggesting that mTOR binding might be insensitive to inhibition with rapamycin.
Figure 3. mTOR binding and activation of Npm1 promoter. (A) Endogenous mTOR is associated with Npm1 promoter region in a rapamycin-independent manner. ChIP assays were conducted using a mTOR antibody in WT and Pten KO MEFs in the absence or the presence of 20 nM rapamycin for 4 hours. Binding of mTOR to Npm1 promoter and Pol-III transcribed tRNAArg/Tyr were determined with PCR primer sets described in Material and Methods. Rabbit IgG were used as negative control. (B) Npm1 is a transcriptional target of mTOR. HeLa (left) and MEF (middle) cell lines were co-transfected for 48 hours with 200 ng of hNPM1-luc plasmid encoding the luciferase under the control of the human NPM1 promoter region and increasing amounts of a pCMV-Flag mTOR vector (100, 200 and 500 ng). The luciferase activity measured in WT MEF cells transfected with hNPM1-luc or CMV-luc was normalized to 1 and a relative fold-induced luciferase activity was then calculated for Pten KO MEFs in the same experimental conditions (right). Error bars represent ± SEM (n = 3; asterisk, p < 0.05; two asterisks, p < 0.01; three asterisks, p < 0.001).
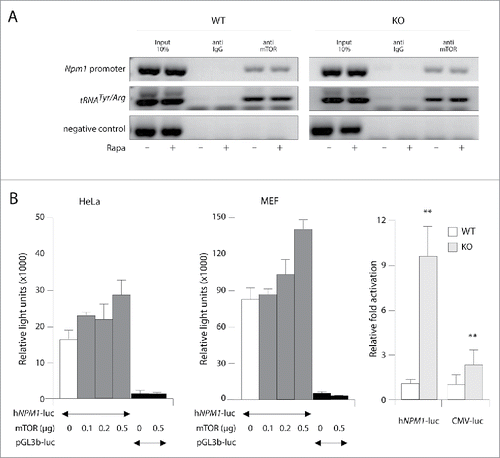
The presence of mTOR at Npm1 template may allow it to regulate transcription of this gene directly. This possibility was explored using the human NPM1 promoter 5′flanking region from −1200 to +87 fused to the luciferase reporter gene. The resulting hNPM1-luciferase construct was transiently transfected into HeLa and MEF cells either alone or in combination with increasing amounts of mTOR expression vector (provided by T. Maeda, University of Tokyo). Following transfection, cells were maintained in serum-free media and 24 h later, cells were harvested and luciferase expression was measured. Ectopic expression of mTOR in HeLa and MEF cells nearly double the NPM1 promoter activity in a dose dependent manner in our experiment conditions (). In addition, we found that the luciferase activity was strongly enhanced in Pten-null MEFs (9.5-fold, p < 0.01) in comparison with wild-type MEFs (). In comparison, cells were transfected with a CMV-driven luciferase expression vector (pcDNA4/T0-luc provided by H Kleinert). As shown in (right panel) the activity from the hNPM1-luciferase reporter gene in Pten deleted MEFs was 4.6-fold more stimulated when compared to that of the CMV-luciferase control gene (p = 0.01). This latter result in combination with the data from our ChIP assays suggest that the assembly of chromatin bounded mTOR competent transcriptional complexes might be blocked by rapamycin since mTOR is not dislodged. In line with this assumption, we did not observe any substantial increase either in the association of mTOR with the endogenous Npm1 promoter in MEF cells inactivated for Pten (). Altogether, these results clearly indicate that mTOR increases the transcriptional activity of the Npm1 promoter although we cannot conclude whether this association requires other transcriptional co-regulators or component of the mTORC1/2 complexes.
Rapamycin regulates Npm1 steady-state mRNA levels
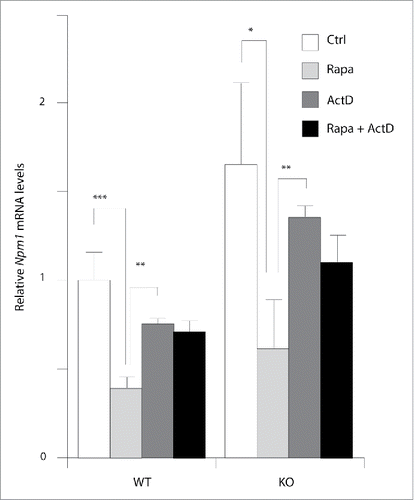
The work described above shows that rapamycin caused a rapid significant decreased in Npm1 mRNA levels in both wild-type and Pten−/− MEF cells at 4 h () suggesting that inhibition of mTOR signaling might affect gene transcription and/or mRNA turnover to regulate Npm1 gene expression. This latter assumption is supported by several lines of evidence showing that signaling through mTOR promotes mRNA decay.Citation27 To test this possibility, the levels of Npm1 mRNA were thus quantified by RT-qPCR in MEF cells in the presence of the RNA polymerase II inhibitor actinomycin D (ActD) or with rapamycin. As shown in , mTOR inactivation by rapamycin down-regulates Npm1 mRNA by around 60% in both wild-type (0.41 ± 0.04 of the control vehicle value, p < 0.001) and Pten deleted MEFs (0.64 ± 0.29 of the control vehicle value, p < 0.05). In contrast, ActD alone only decreased mRNA levels from 25% in both cell lines suggesting that inhibition with rapamycin strongly upregulates Npm1 mRNA turnover. Of note, addition of ActD to cells treated with rapamycin prevents the effect observed with rapamycin alone. Thus, the enhanced turnover of Npm1 mRNA in mTOR-inhibited cells can be suppressed by inhibition of RNA polymerase II transcription. This may reflect the need of a transcriptional event to recruit the general cellular mRNA degradation machinery to regulate Npm1 mRNA turnover.
Figure 4. mTOR signaling inhibition by rapamycin increase Npm1 mRNA turnover. Total mRNA from early log phase growing MEFs were used for qPCR analysis of Npm1 expression. Cells were treated with actinomycin D (5µg/ml), rapamycin (20 nM), or rapamycin plus actinomycin D as indicated. Cells were harvested after 10 h and total mRNA was extracted for Npm1 expression evaluation. The mRNA level in WT MEFs alternatively treated with vehicle was considered as 100%. Data are representative of 3 independent experiments and expressed as fold change relative to WT vehicle-treated MEFs, taken as calibrator for comparative quantitation analysis of mRNA levels. Each sample was measured in triplicate and bar graphs represent mean ± SEM (*p < 0.05; **p < 0.01; ***p < 0.001).
Npm1 is a downstream effector of mTOR signaling with implication in prostate cancer
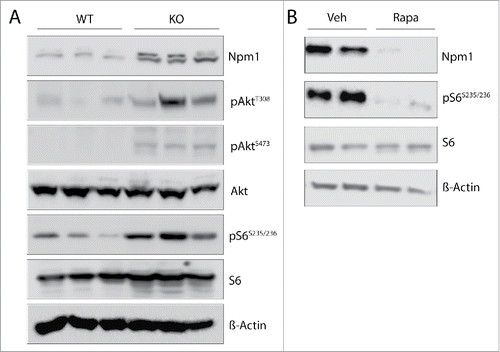
Our previous report that NPM1 is overexpressed in human samples of prostate cancer,Citation21 together with the fact that mTOR serves a pivotal role in cancer suggest that these two proteins may be functionally linked to regulate events involved in prostate cancer cell growth control. To answer this, we used the conditional murine Pten prostate cancer model with a constitutive activation of the PI3K/Akt/mTOR signaling pathway in the prostate epithelium.Citation23 This model is greatly appropriate since it recapitulates the disease progression seen in humans with the formation of high-grade prostatic intraepithelial neoplasia and invasive carcinoma. Consistent with our findings in MEF cells deleted for Pten, the abundance of the Npm1 protein is increased in prostate tumors of Pten−/− animals in conjunction with Akt activation and phosphorylation of the downstream mTOR effector S6 ribosomal protein (). Of note, chronical administration for 4 days with rapamycin (10 mg/kg/day) almost completely inhibits the expression of Npm1 in the dorsolateral compartment of prostate gland of Pten knockout mice (). The lack of phosphorylation of S6 protein in rapamycin-treated animals confirms that mTOR was efficiently blocked by our treatment and let us conclude that mTOR also regulates Npm1 expression in vivo.
Figure 5. Npm1 overexpression in mouse prostate tumors induced by Pten loss is sensitive to chronic mTOR inhibition by rapamycin. (A) Homozygous Pten deletion increases Npm1 abundance in the mouse dorsolateral prostate gland lobe. Protein lysates were prepared from three month-old animals and were probed with the indicated antibodies by western immunoblotting. (B) Chronic mTOR inhibition decreases the abundance of Npm1 in Pten-null prostate cancer. Three-month old Pten conditional knockout mice were chronically administrated with either vehicle or rapamyscin (10 mg/kg/day). After 4 days, protein levels in the dorsolateral compartment of the Pten-null prostate lobe were separated by SDS-PAGE and assayed by western blotting using the indicated antibodies. Representative blots are derived from 2 different animals per group. Compared Pten knockout mice treated with vehicle (lanes 1 and 2) with rapamycin-treated animals lanes 3 and 4).
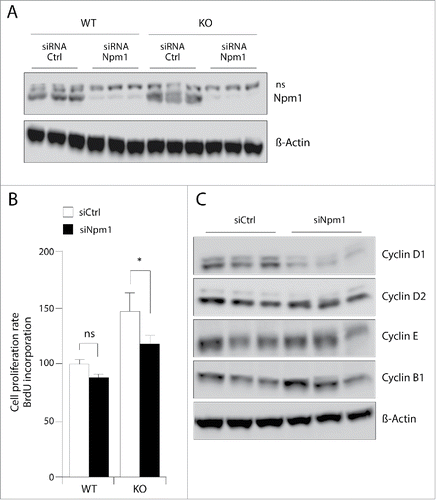
One important remaining question is to know whether Npm1 acts as a downstream mediator of mTOR to promote cellular adaptation for growth. This questioning cannot be solved by deleting Npm1 gene expression in the Pten knockout mice because its complete inactivation leads to embryonic lethality at mid-gestation,Citation28 and no mouse model for conditional inactivation of Npm1 is currently available. To circumvent this limitation, we have assessed the proliferation rate of wild-type and Pten−/− MEFs, non-silenced or silenced for Npm1, using the bromodeoxyuridine (BrdU) incorporation cell-based assay. As shown in , Npm1 silencing efficiency was near 90% compared to control cultures transfected with a non-specific control unrelated GFP siRNA sequence (). Interestingly, the growth advantage of Pten−/− MEFs is significantly reduced after depletion of Npm1 (20% inhibition, p < 0.05) while Npm1 downregulation had a moderate effect on the proliferation of control wild-type MEFs (). When compared to control wild-type MEFs, Npm1 silencing prevents the growth enhancement induced by Pten loss. One possible explanation for this may reside in the decreased expression of cyclin D1 in Pten inactivated MEFs silenced for Npm1 (). This is an interesting finding since Pten acts as general negative regulator of cyclin D expression.Citation29 Indeed, inhibiting Npm1 in MEF cells with hyperactive Akt/mTOR signaling may thus contribute to counteract the increased expression of cyclin D1 in Pten inactivated MEFs and to restore cell cycle regulation in these cells. Collectively, these results suggest that Npm1 may function downstream of mTOR to coordinate mitogenic signals to control cell growth and cell-cycle progression.
Figure 6. Pten deletion-induced proliferation in MEFs with hyperactive mTOR requires Npm1. (A) Exponentially growing MEFs were transfected with specific Npm1 or control GFP (Ctrl) siRNAs. 24 hours after transfection with 50 nM Npm1 and Ctrl GFP siRNAs, whole-cell extracts were prepared and protein gel blot was performed using antibodies against Npm1 and β-Actin for loading control. (B) Npm1 depletion reverses Pten-loss induced hyper-proliferation of MEFs with hyperactive mTOR signaling. Wild-type and inactivated MEFs for Pten were plated at a respective density of 17,5 × 103 and 7,5 × 103 cell per well into a 96-wells plate and were transfected 1 day later with either Npm1 and Ctrl GFP siRNAs. After 24 hours, medium was changed and the cell proliferation was assayed by using the BrdU reagent according to the Material and Methods. (C) Npm1 silencing decreased the upregulated Cyclin D1 protein level induced by Pten inactivation. MEFs deleted for Pten were transfected as above and total protein lysate of equal numbers of cells were subjected to Western blot analysis with antibodies to cyclin D1, cyclin D2, cyclin E, and cyclin B1. β-Actin levels are shown as a loading control. (n = 3) *p < 0.05.
Discussion
Signaling through the mammalian target of rapamycin (mTOR) regulates many diverse cellular processes, especially those contributing to cell growth and proliferation. In the past couple of year, many important advances have been made in identifying new components of the mTOR network and in further elucidating connections between known components. In the present study, we provide evidence that Npm1 is a downstream signaling target of mTOR that may participate in the mTOR-dependent coordination of cell growth and cell-cycle progression in response to mitogenic signals. By actively shuttling between the nucleolus and cytoplasm, Npm1 was reported too to take on various distinct cellular functions. Indeed, the biological significance of Npm1 in ribosome biogenesis and transport, anti-apoptotic activity, the regulation of centrosome duplication and the regulation of tumors suppressors have been largely documented although its upstream regulatory mechanisms still represent a major gap to fulfill.Citation16,30-32
Using cell lines and animal models bearing a targeted deletion of the Pten gene that leads to hyperactivation of the PI3K/Akt/mTOR signaling, we find that Pten-null MEFs display more Npm1 protein and mRNA than their wild-type counterpart. This increase is also observed in the prostate gland of the conditional murine Pten cancer model indicating thus that active mTOR signaling pathway in prostate cancer cells might also impinge on Npm1 gene expression in vivo. In both situations, Npm1 enhanced expression is sensitive to rapamicyn inhibition of mTOR although our in vitro data show that mTOR association with the Npm1 promoter is insensitive to rapamycin. Actually, it is not clear how this kinase acts to increase the transcriptional activity we observed toward the Npm1 promoter and we cannot exclude that this later may serve to assemble competent transcriptional mTORC complexes onto chromatin. Of interest, the mTOR enriched 5′ enhancer promoter region of Npm1 includes a functional YY1-binding motif that has been already characterized.Citation17 It is thus tempting to suggest that mTOR might control Npm1 gene transcription through a YY1 transcriptional complex as reported for other genes that adapts the mitochondrial oxidative function in response to nutrients and hormonal signals.Citation19 This should be easily assessed by testing the mTOR-YY1 interaction into chromatin to determine whether this interface constitute a key regulatory step to enhance Npm1 gene expression when mTOR is hyperactive. Moreover, silencing YY1 as well as components of the TORC complexes namely the Raptor and Rictor proteins may provide additional valuable insights to unravel the mechanism of Npm1 gene transcriptional activation by mTOR dependent complexes.
Interestingly, mTOR binds to promoters of RNA polymerase I- and III- transcribed genes and these associations with rDNA (rDNA) and tRNA (tRNA) enhancer regions are believed to be crucial to the protein synthetic capacity of the cell and its growth ability. By directly controlling Npm1 gene expression, mTOR would allow the cell to connect nutrient and/or hormonal signals to activate ribosome biogenesis. This is a particularly interesting point because Npm1 was initially described as a ribosome chaperone or assembly factor that was found associated with pre-rRNA processing. In support of this notion, a fraction of soluble Npm1 interacts with pre-ribosomal 60S particles,Citation33 facilitates cleavage of rRNA in vitro and acts as an endoribonuclease for the maturing rRNA transcript.Citation34,35 Not surprisingly, inhibition of Npm1 thus results in a decrease in cell growth as a consequence of impaired processing of pre-rRNA to mature 28S rRNA and inhibition of ribosome subunit export from the nucleolus to the cytoplasm.Citation36,37 Altogether, these observations support the notion of a functional coupling between mTOR and Npm1 to coordinate the manufacturing of ribosomes and our finding that rapamycin down-regulates Npm1 abundance may provide an explanation to understand why mTOR signaling inhibition interferes with the processing of rRNA as recently noted by Iadevaia et al.Citation38
The regulation of Npm1 mRNA turnover we have reported in this study constitutes an unexpected finding that raises important questions in regards to the specific elements within the Npm1 mRNA that controls its stability and the cytoplasmic mRNA degradation machinery that might be activated when mTOR pathway is inhibited. In fact, multiples mRNAs were demonstrated to be destabilized after treatment with rapamycin in yeastCitation27 and our findings now define the Npm1 mRNA as a new potential target for the mTOR regulated mRNA decay. From our complementary experiments, Npm1 mRNA destabilization is unlikely to occur through the AU-rich-mediated decay machinery which has been recently described by Cammas et al.Citation39 While in muscle cells the AU-rich elements (AREs) located at the 3′ untranslated region (3′-UTR) sequence of the Npm1 mRNA were shown to participate to the posttranscriptional downregulation of the Npm1 gene expression, inhibition of mTOR by rapamycin did not destabilizes a Renilla luciferase reporter gene fused to the Npm1-3′-UTR sequence (Rluc-NPM-3′ provided by A Cammas) in transiently transfected MEF cells (not shown). This allows us to conclude that the destabilization of Npm1 mRNA may be regulated through another mechanism of mRNA decay, and the actinomycin D-mediated stabilization of Npm1 mRNA in presence of rapamycin suggests the need of a RNA pol II-dependent transcription event to control Npm1 mRNA stability. One attracting possibility is the transcriptional repression of antisense non-coding RNA (ncRNA) by mTOR dependent complexes. This hypothesis fits well with our data, since inhibition of mTOR by rapamycin could relieve the transcriptional repression of ncRNAs and allow their complementary association with Npm1 mRNA for targeted degradation. In this sense, mRNA degradation by microRNA (miRNA), small interfering RNA (siRNA) and large intergenic non-coding RNA (lncRNA) all appears as plausible possibilities and inhibition of RNA pol-II transcription by actinomycin D should stabilize Npm1 mRNA in presence of rapamycin. This is a very interesting issue to address and further work is required to completely understand how mTOR pathway is post-transcriptionally regulating Npm1 gene expression.
Nevertheless, this information combined with the results we provided herein may have important implications for cancer biology since mTOR is a critical effector of cell-signaling pathways which is commonly deregulated in human malignancies.Citation40 This is particularly the case in numerous human primary prostate cancers and in high-grade prostatic intra-epithelial neoplasias with Pten genomic deletions.Citation41,42 Indeed, several molecules within the PI3K/AKT/mTOR signaling pathway can be altered during prostate cancer progression with potential diagnostic markers including PTEN, p-AKT, and mTOR.Citation43,44 In addition, mTOR signaling molecules have been especially proposed as central players in prostate cancer because of their influence on cell growth capacities.Citation45 The general belief is that activated mTOR may provide certain tumors cells with a growth advantage by promoting protein synthesis, which is the best-described function of mTOR. By regulating Npm1 gene expression through transcriptional and posttranscriptional mechanisms, increased mTOR activity may lead to the enhance Npm1 expression that we and others already reported in human prostate cancer.Citation21,46 It is currently unknown whether deregulated mTOR and Npm1 are associated in clinical samples of prostate cancer but it is tempting to speculate that mTOR might stabilize Npm1 mRNA levels to stimulate cell growth by regulating protein synthesis and cell cycle progression. The increased abundance of Npm1 in Pten knockout mouse prostate cancer model is in good agreement with this assumption and the possibility to restore normal cell cycle progression in Pten inactivated MEF cells silenced for Npm1 further strengthens the possibility of its functional role as a downstream effector of mTOR signaling. Indeed, our ongoing analysis of NPM1 in human prostate tissue microarrays and its correlation with PI3K/AKT/mTOR activation should help to clarify the importance of mTOR in NPM1 dysregulation. Moreover, it should confirm the spearman correlation test we performed using the GSE 35988 dataset from Grasso et alCitation47 (see Fig. S2) that is showing a positive association between both Npm1 and mTOR mRNA levels in sample of metastatic prostate cancer (Spearman test, R = 04137; p = 0.0135). Complementary analyses are still required to fully assess the role of mTOR in the regulation of Npm1 gene expression in prostate cancer disease. Nevertheless, the data described herein report for the first time that Npm1 acts as a downstream effector of mTOR signaling to regulate cell proliferation with potential implications for prostate cancer and its therapy.
Materials and methods
Chemicals and biochemicals
Life Technologies supplies the Dulbecco's modified Eagle medium (DMEM, cat#41965), glutamine (cat#25030), antibiotics (Pen/Strep, cat#15140), DPBS 10X (cat#14200). Fetal bovine serum was obtained from Biowest (cat#51810). Rapamycin and the non-ionic emulsifier Cremephor EL were respectively purchased from LC Laboratories (#catR5000) and Sigma-Aldrich (#catC5135). Goat polyclonal anti-NPM1 (C-19, sc-6013), rabbit polyclonal anti-cyclin D1 (M20, sc-718), rabbit polyclonal anti-cyclin E (M-20, sc-481), rabbit polyclonal anti-cyclin A (C-19, sc-596) and rabbit polyclonal anti-cyclin B1 (H-433, sc-752) were purchased from Santa Cruz Biotechnology; rabbit polyclonal anti-phospho-AKT Ser473 (ab81283) from AbCam, rabbit monoclonal anti-phospho-AKT Thr308 (cat#2965), rabbit monoclonal anti-AKT (cat#9272), rabbit monoclonal anti-S6 (cat#2217), rabbit polyclonal anti-phospo-S6 Ser235/236 (cat#2211), rabbit polyclonal anti-mTOR (cat#2972) from cell signaling; and rabbit polyclonal anti-βactin from Sigma-Aldrich (cat#A2066). The hNPM1-luciferase reporter gene was obtained by cloning the PCR amplified DNA fragment from the −1200 to +86 promoter region of the human NPM1 gene (Forward primer, 5′-GATCGGTACCGTGACACAGGAGCTCTCAGAAAGG-3′; Reverse primer 5′-GATCCTCGAGACTTAGGTAGGAGAGAAGGCGGAC-3′) directly into the KpnI/XhoI sites of the pGL3-basic luciferase reporter vector (Promega).
Cell cultures and transfections
Mouse Embryonic Fibroblast cells were cultured in 10 cm plates in DMEM supplemented with 10% fetal calf serum at 37°C in a humidified atmosphere containing 5% CO2. HeLa cells were maintained in DMEM supplemented with 10% fetal calf serum, as above. For transient transfections, 3 × 105 HeLa cells per well were plated in 6 well-plates and the next day, transient transfections were performed using the Metafecten procedure (Biontex Laboratories GmbH). For wild-type and Pten knockout MEFs, 2 × 105 and 1 × 105 cells/well were plated respectively and cells were transfected the next day according to the same procedure. After 12 h, the medium was replaced with fresh complete medium and 2 days later, cells were lysed and the luciferase activity was measured with the assay system from Yelen. For depletion of Npm1 and mTOR, we used the siGenome mouse Npm1 (cat#18148) and mouse mTOR (cat#56717) siRNA- SMART pools from Dharmacon (sequences available upon request).
Animals and treatments
Mice were housed individually (24°C; light on 07.00–19.00 h) and allowed free access to water and diet (A03 standard diet cat#U8200G10R from SAFE). When stated, animals received daily i.p. injections of either vehicle for the control or Sirolimus (rapamycin) at a dose of 10 mg/kg per day for 4 days. Mice were killed by cervical dislocation and tissues were freeze-clamped and stored at −80°C for further analyses. All animal care and experimental procedures were in accordance with the French guidelines for animal experimentation and were ethically approved by the local C2E2A committee on Animal Experiments.
RNA extraction and RT-qPCR analysis
Total RNA was isolated from MEF cells using the RNAzol method according to the manufacturer's instructions (Quantum). For cDNA synthesis, five hundred nanograms mRNA were reverse transcribed for 1 h at 42°C with 5pmoles of random hexamer primers, 200 units reverse transcriptase (MuMLV RT, Promega), 2 mM dNTPs and 20 units RNAsin (Promega) as described.Citation48 A half microliter of one-tenth dilution of the cDNA was used in each quantitative PCR reaction. These were conducted with the mouse Npm1 sequence specific primers (Forward: 5′-GCAGGGGCAAAAGATGAGT-3′; Reverse: 5′-CAAAGCCCCCTAGGGAAAC). Each reaction was performed in triplicate in a final volume of 15µl with 0.75µl of the appropriate 20X probe mix and 7.5µl of PCR Mastermix (Precision-iC., PrimerDesign.co.uk). Relative mRNA accumulation was determined by the ΔΔCt method and 36b4 (Forward: 5′-GTCACTGTGCCAGCTCAGAA-3′, Reverse: 5′-TCAATGGTGCCTCTGGAGAT-3′) was used for quantative normalization of cDNA in each cell-derived sample.
Preparation of cell extracts and western blot analysis
Mouse tissues were harvested from three-month old wild-type and PTENLoxP/LoxP; PB-Cre4+ mice and immediately homogenized in ice cold NaCl buffer [0.42 M NaCl, 20 mM Hepes (pH 8.0), 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 0.1% NP40, 1 mM PMSF, 1μg/ml apoprotinin, 1μg/ml leupeptin]. To make the whole-cell lysates, MEF cells were washed with 1X PBS and resuspended in ice cold NaCl buffer. After brief sonication on ice and clarification for 30 min at 15,000 × g, 40µg of proteins were resolved through SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked with milk buffer [5% milk in Tris-buffered saline (TBS)-Tween 20] for 1 h and incubated with the indicated primary antibody in milk buffer overnight at 4°C. The membranes were washed three times with TBS-Tween 20 and then incubated 90 min with horseradish peroxidase-conjugated donkey anti-rabbit or goat anti-rabbit immunoglobulin G secondary antibody (P.A.R.I.S biotech), followed by enhanced chemiluminescence (Santa Cruz Biotechnology) according to manufacturer's instruction.
Cell proliferation assay
Cell proliferation was determined using the cell-based BrdU (Bromode oxyuridine) proliferation assay. Briefly, MEF cells were seeded in 96-wells plate and transfected with 50 nM specific Npm1 or control GFP siRNAs. The next day, medium was changed and cells were cultured in 200µl of complete medium. After 48 hours, the BrdU labeling solution was added at a final concentration of 10 mM for 2.5 hours and the absorbance was measured at 655 nm following fixation after an additional incubation of 1 hour with the anti BrdU-POD solution according to the Cell Proliferation ELISA BrdU colorimetric kit from Roche Applied Science.
Chromatin immunoprecipitation
Chromatin immunoprecipitation assays were performed according to the Upstate protocol. Sheared DNA fragments were immunoprecipitated with an anti-mTOR (Abcam, ab32028) and a mock rabbit IgG (Diagenode) was used as a negative control. Sequences of primers used for mouse Npm1 promoter PCR were: Forward 5′-GCAGGGGCAAAAGATGAGT-3′ and Reverse 5′-CAAAGCCCCCTAGGGAAAC). Primer sets for mouse tRNAArg/Tyr (chr3 segment 19528000 to 19528500: Forward 5′- CTTGCCGCTCCCTTCGATAG-3′; Reverse 5′-AGAGGAGGAGGAGCGCACCTG-3′) and a negative control non-mTOR-bound genomic region (Forward 5′-TTGGCATTGATATTGGGGGTGGGAGCAACT-3′; Reverse 5′ GACTTCTTACTTTGACGCTTTCCTCCA-3′) were used as described.Citation7 PCR conditions for all primers were as follows: 94°C for 3 min, followed by 32–35 cycles at 94°C for 30s, 55–58°C for 45s and 72°C for 45s.
Statistical analyses
All experiments were repeated at least three times and results were expressed as mean ± SEM. Statistical differences between groups were determined by Student's t test and the criterion for statistical significance was p < 0,05. For protein gel blotting results, the band intensities were measured by using the ImageJ and normalized with β-Actin.
Abbreviations
| mTOR | = | mammalian target of rapamycin |
| Npm1/B23 | = | nucleophosmin |
| MEF | = | murine embryo fibroblast |
| SiRNA | = | small interfering RNA |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
2015CC6826R1-f08-z-bw.pdf
Download PDF (153.4 KB)2015CC6826R1-f07-z-bw.pdf
Download PDF (530.4 KB)Acknowledgments
Authors gratefully acknowledge Angélique De Haze for help in various phases of the experimental work and are obliged to the members of the CNRS UMR6293-GReD for helpful discussion and critical reading during the preparation of the manuscript.
Funding
This work was granted by the Centre National de la Recherche Scientifique (CNRS), the Blaise Pascal University, the Institut National de la Santé et de la Recherche Médicale (INSERM), and the CLARA Cancer Auvergne Prostate program (CVPPRCAN000145). RB is holder of a studentship from the French Foundation for Medical Research (FRM).
References
- Aramburu J, Ortells MC, Tejedor S, Buxade M, Lopez-Rodriguez C. Transcriptional regulation of the stress response by mTOR. Sci Signal 2014; 7:re2; PMID:24985347; http://dx.doi.org/10.1126/scisignal.2005326
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274-93; PMID:22500797; http://dx.doi.org/10.1016/j.cell.2012.03.017
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 2002; 110:177-89; PMID:12150926; http://dx.doi.org/10.1016/S0092-8674(02)00833-4
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 2004; 14:1296-302; PMID:15268862; http://dx.doi.org/10.1016/j.cub.2004.06.054
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 2009; 10:307-18; PMID:19339977; http://dx.doi.org/10.1038/nrm2672
- Tsang CK, Liu H, Zheng XF. mTOR binds to the promoters of RNA polymerase I- and III-transcribed genes. Cell Cycle 2010; 9:953-7; PMID:20038818; http://dx.doi.org/10.4161/cc.9.5.10876
- Chaveroux C, Eichner LJ, Dufour CR, Shatnawi A, Khoutorsky A, Bourque G, Sonenberg N, Giguere V. Molecular and genetic crosstalks between mTOR and ERRalpha are key determinants of rapamycin-induced nonalcoholic fatty liver. Cell Metab 2013; 17:586-98; PMID:23562079; http://dx.doi.org/10.1016/j.cmet.2013.03.003
- Feuerstein N, Mond JJ. “Numatrin,” a nuclear matrix protein associated with induction of proliferation in B lymphocytes. J Biol Chem 1987; 262:11389-97; PMID:3301855
- Okuwaki M. The structure and functions of NPM1/Nucleophsmin/B23, a multifunctional nucleolar acidic protein. J Biochem 2008; 143:441-8; PMID:18024471; http://dx.doi.org/10.1093/jb/mvm222
- Johansson H, Simonsson S. Core transcription factors, Oct4, Sox2 and Nanog, individually form complexes with nucleophosmin (Npm1) to control embryonic stem (ES) cell fate determination. Aging (Albany NY) 2010; 2:815-22; PMID:21076177; http://dx.doi.org/10.18632/aging.100222
- Chan WY, Liu QR, Borjigin J, Busch H, Rennert OM, Tease LA, Chan PK. Characterization of the cDNA encoding human nucleophosmin and studies of its role in normal and abnormal growth. Biochem 1989; 28:1033-9; http://dx.doi.org/10.1021/bi00429a017
- Feuerstein N, Chan PK, Mond JJ. Identification of numatrin, the nuclear matrix protein associated with induction of mitogenesis, as the nucleolar protein B23. Implication for the role of the nucleolus in early transduction of mitogenic signals. J Biol Chem 1988; 263:10608-12; PMID:3392030
- Li J, Zhang X, Sejas DP, Bagby GC, Pang Q. Hypoxia-induced nucleophosmin protects cell death through inhibition of p53. J Biol Chem 2004; 279:41275-9; PMID:15310764; http://dx.doi.org/10.1074/jbc.C400297200
- Wu MH, Chang JH, Chou CC, Yung BY. Involvement of nucleophosmin/B23 in the response of HeLa cells to UV irradiation. Int J Cancer 2002; 97:297-305; PMID:11774280; http://dx.doi.org/10.1002/ijc.1606
- Wu MH, Yung BY. UV stimulation of nucleophosmin/B23 expression is an immediate-early gene response induced by damaged DNA. J Biol Chem 2002; 277:48234-40; PMID:12374805; http://dx.doi.org/10.1074/jbc.M206550200
- Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer 2006; 6:493-505; PMID:16794633; http://dx.doi.org/10.1038/nrc1885
- Chan PK, Chan FY, Morris SW, Xie Z. Isolation and characterization of the human nucleophosmin/B23 (NPM) gene: identification of the YY1 binding site at the 5′ enhancer region. Nucleic Acids Res 1997; 25:1225-32; PMID:9092633; http://dx.doi.org/10.1093/nar/25.6.1225
- Zeller KI, Haggerty TJ, Barrett JF, Guo Q, Wonsey DR, Dang CV. Characterization of nucleophosmin (B23) as a Myc target by scanning chromatin immunoprecipitation. J Biol Chem 2001; 276:48285-91; PMID:11604407
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 2007; 450:736-40; PMID:18046414; http://dx.doi.org/10.1038/nature06322
- Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 2010; 39:171-83; PMID:20670887; http://dx.doi.org/10.1016/j.molcel.2010.06.022
- Leotoing L, Meunier L, Manin M, Mauduit C, Decaussin M, Verrijdt G, Claessens F, Benahmed M, Veyssiere G, Morel L, et al. Influence of nucleophosmin/B23 on DNA binding and transcriptional activity of the androgen receptor in prostate cancer cell. Oncogene 2008; 27:2858-67; PMID:18037965; http://dx.doi.org/10.1038/sj.onc.1210942
- Loubeau G, Boudra R, Maquaire S, Lours-Calet C, Beaudoin C, Verrelle P, Morel L. NPM1 silencing reduces tumour growth and MAPK signalling in prostate cancer cells. PloS One 2014; 9:e96293; PMID:24796332; http://dx.doi.org/10.1371/journal.pone.0096293
- Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 2003; 4:209-21; PMID:14522255; http://dx.doi.org/10.1016/S1535-6108(03)00215-0
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 2009; 122:3589-94; PMID:19812304; http://dx.doi.org/10.1242/jcs.051011
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 2006; 22:159-68; PMID:16603397; http://dx.doi.org/10.1016/j.molcel.2006.03.029
- Pelletier CL, Maggi LB, Jr, Brady SN, Scheidenhelm DK, Gutmann DH, Weber JD. TSC1 sets the rate of ribosome export and protein synthesis through nucleophosmin translation. Cancer Res 2007; 67:1609-17; PMID:17308101; http://dx.doi.org/10.1158/0008-5472.CAN-06-2875
- Albig AR, Decker CJ. The target of rapamycin signaling pathway regulates mRNA turnover in the yeast Saccharomyces cerevisiae. Mol Biol Cell 2001; 12:3428-38; PMID:11694578; http://dx.doi.org/10.1091/mbc.12.11.3428
- Grisendi S, Bernardi R, Rossi M, Cheng K, Khandker L, Manova K, Pandolfi PP. Role of nucleophosmin in embryonic development and tumorigenesis. Nature 2005; 437:147-53; PMID:16007073; http://dx.doi.org/10.1038/nature03915
- Diao L, Chen YG. PTEN, a general negative regulator of cyclin D expression. Cell Res 2007; 17:291-2; PMID:17426697; http://dx.doi.org/10.1038/cr.2007.24
- Lim MJ, Wang XW. Nucleophosmin and human cancer. Cancer Detect Prev 2006; 30:481-90; PMID:17113241; http://dx.doi.org/10.1016/j.cdp.2006.10.008
- Lindstrom MS. NPM1/B23: A Multifunctional Chaperone in Ribosome Biogenesis and Chromatin Remodeling. Biochem Res Int 2011; 2011:195209; PMID:21152184; http://dx.doi.org/10.1155/2011/195209
- Yung BY. Oncogenic role of nucleophosmin/B23. Chang Gung Med J 2007; 30:285-93; PMID:17939258
- Yu Y, Maggi LB, Jr, Brady SN, Apicelli AJ, Dai MS, Lu H, Weber JD. Nucleophosmin is essential for ribosomal protein L5 nuclear export. Mol Cell Biol 2006; 26:3798-809; PMID:16648475; http://dx.doi.org/10.1128/MCB.26.10.3798-3809.2006
- Savkur RS, Olson MO. Preferential cleavage in pre-ribosomal RNA byprotein B23 endoribonuclease. Nucleic Acids Res 1998; 26:4508-15; PMID:9742256; http://dx.doi.org/10.1093/nar/26.19.4508
- Szebeni A, Herrera JE, Olson MO. Interaction of nucleolar protein B23 with peptides related to nuclear localization signals. Biochemistry 1995; 34:8037-42; PMID:7794916; http://dx.doi.org/10.1021/bi00025a009
- Itahana K, Bhat KP, Jin A, Itahana Y, Hawke D, Kobayashi R, Zhang Y. Tumor suppressor ARF degrades B23, a nucleolar protein involved in ribosome biogenesis and cell proliferation. Mol Cell 2003; 12:1151-64; PMID:14636574; http://dx.doi.org/10.1016/S1097-2765(03)00431-3
- Maggi LB, Jr, Kuchenruether M, Dadey DY, Schwope RM, Grisendi S, Townsend RR, Pandolfi PP, Weber JD. Nucleophosmin serves as a rate-limiting nuclear export chaperone for the Mammalian ribosome. Mol Cell Biol 2008; 28:7050-65; PMID:18809582; http://dx.doi.org/10.1128/MCB.01548-07
- Iadevaia V, Zhang Z, Jan E, Proud CG. mTOR signaling regulates the processing of pre-rRNA in human cells. Nucleic Acids Res 2012; 40:2527-39; PMID:22121221; http://dx.doi.org/10.1093/nar/gkr1040
- Cammas A, Sanchez BJ, Lian XJ, Dormoy-Raclet V, van der Giessen K, Lopez de Silanes I, Ma J, Wilusz C, Richardson J, Gorospe M, et al. Destabilization of nucleophosmin mRNA by the HuR/KSRP complex is required for muscle fibre formation. Nat Commun 2014; 5:4190; PMID:24969639; http://dx.doi.org/10.1038/ncomms5190
- Wang X, Huang H, Young KH. The PTEN tumor suppressor gene and its role in lymphoma pathogenesis. Aging (Albany NY) 2015; 7:1032-49; PMID:26655726; http://dx.doi.org/10.18632/aging.100855
- Yoshimoto M, Cutz JC, Nuin PA, Joshua AM, Bayani J, Evans AJ, Zielenska M, Squire JA. Interphase FISH analysis of PTEN in histologic sections shows genomic deletions in 68% of primary prostate cancer and 23% of high-grade prostatic intra-epithelial neoplasias. Cancer Genet Cytogenet 2006; 169:128-37; PMID:16938570; http://dx.doi.org/10.1016/j.cancergencyto.2006.04.003
- McCall P, Witton CJ, Grimsley S, Nielsen KV, Edwards J. Is PTEN loss associated with clinical outcome measures in human prostate cancer? Br J Cancer 2008; 99:1296-301; PMID:18854827; http://dx.doi.org/10.1038/sj.bjc.6604680
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res 1997; 57:4997-5000; PMID:9371490
- Majumder PK, Yeh JJ, George DJ, Febbo PG, Kum J, Xue Q, Bikoff R, Ma H, Kantoff PW, Golub TR, et al. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci U S A 2003; 100:7841-6; PMID:12799464; http://dx.doi.org/10.1073/pnas.1232229100
- Kremer CL, Klein RR, Mendelson J, Browne W, Samadzedeh LK, Vanpatten K, Highstrom L, Pestano GA, Nagle RB. Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate 2006; 66:1203-12; PMID:16652388; http://dx.doi.org/10.1002/pros.20410
- Subong EN, Shue MJ, Epstein JI, Briggman JV, Chan PK, Partin AW. Monoclonal antibody to prostate cancer nuclear matrix protein (PRO:4-216) recognizes nucleophosmin/B23. Prostate 1999; 39:298-304; PMID:10344220; http://dx.doi.org/10.1002/(SICI)1097-0045(19990601)39:4<298::AID-PROS11>3.0.CO;2-M
- Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012; 487:239-43; PMID:22722839; http://dx.doi.org/10.1038/nature11125
- Berthon A, Sahut-Barnola I, Lambert-Langlais S, de Joussineau C, Damon-Soubeyrand C, Louiset E, Taketo MM, Tissier F, Bertherat J, Lefrancois-Martinez AM, et al. Constitutive β-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet 2010; 19:1561-76; PMID:20106872; http://dx.doi.org/10.1093/hmg/ddq029
