Chronic inflammation is associated with a variety of pathological conditions in self-renewing epithelial tissues, including cancer, metaplasia and impaired wound healing, and evidence indicates that it may in fact be an initiating factor for these pathologies.Citation1 Despite evidence of a causative link, the mechanisms by which chronic inflammation promotes disease are only beginning to be elucidated. In this regard, many of the pathologies linked to chronic inflammation are associated with aberrant function of epithelial stem cells, which normally maintain equilibrium during homeostasis or mediate repair following injury. For example, intestinal stem cells exhibit hyperproliferation in patients with inflammatory bowel disease, who are also at increased risk of developing bowel cancer.Citation2 Thus, understanding the cellular and molecular interactions that occur between the chronic inflammatory environment and epithelial stem cells is important for understanding disease and will potentially identify novel therapeutic targets.
Like all adult stem cell populations, tissue resident epithelial stem cells are subject to regulation by a their microenvironment or niche, which consists of a variety of components including mesenchymal cells, vascular endothelial cells and extracellular matrix (ECM) components.Citation3 Collectively, these provide a coordinated and complex array of cues to ensure stem cells function appropriately for the needs of the tissue. During repair or in chronic inflammatory conditions, immune cells infiltrate the local microenvironment and thus have a profound impact on both the niche and stem cell function.
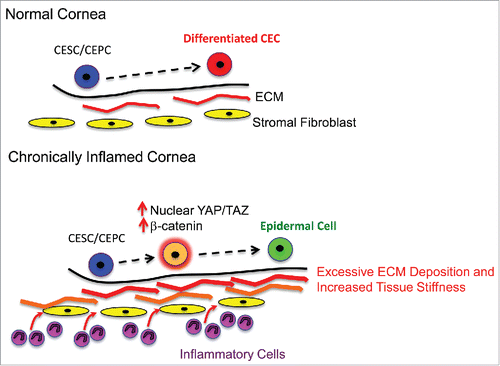
In relation to this, a recent report from our laboratory has revealed an important mechanism by which chronic inflammation promotes abnormal cell fate in regenerating epithelia.Citation4 In this study, we investigated the link between chronic inflammation and a condition known as corneal squamous cell metaplasia (CSCM), in which corneal epithelial cells adopt a keratinizing, epidermal-like fate, resulting in ocular surface blindness. By using genetic mouse models of CSCM we demonstrated that chronic inflammation of the corneal stroma promotes epidermal fate conversion on the ocular surface by inducing excessive deposition of ECM in the local microenvironment. This resulted in increased tissue stiffness and elicited aberrant mechanotransduction in activated epithelial stem/progenitor cells mediating regeneration. As a consequence, a YAP/TAZ-β-catenin cascade was triggered, which promoted entry into the epidermal lineage ().
Figure 1. Chronic inflammation promotes metaplasia on the ocular surface by eliciting aberrant mechanotransduction in stem cells. In the normal cornea, corneal epithelial stem/progenitor cells differentiate into non-keratinizing corneal epithelial cells that form a protective barrier on the anterior ocular surface. Chronic inflammation leads to excessive ECM deposition by stromal fibroblasts, which increases tissue stiffness. This promotes mechanotransduction in stem/progenitor cells resulting in increased nuclear translocation of YAP/TAZ, stabilization of β-catenin and induction of epidermal differentiation. CESC/CEPC – Corneal Epithelial Stem/Progenitor Cell, CEC – Corneal Epithelial Cell, ECM – Extracellular Matrix.
The results of this study therefore identified that an important mechanism by which chronic inflammation can promote aberrant stem cell fate decisions and subsequent pathology is via alteration of tissue mechanical cues. Thus, in addition to regulating stem cell function by secreting biochemical factors, chronic inflammation also influences stem cell behavior by changing the mechanical properties of the stem cell microenvironment. Further elucidation of the link between chronic inflammation, mechanotransduction and stem cell function will yield important insight into the underlying mechanisms of a variety of diseases, including cancer, and will potentially identify novel therapeutic targets. Key questions to be addressed will include deciphering the precise cellular and molecular mechanisms by which chronic inflammation can elicit changes in tissue mechanical properties. In this, respect, a key area of interest will be in understanding the effect of chronic inflammation on the ECM, which directly affects physical properties such as elasticity and topography. Changes in the composition and concentration of ECM components as well as post-translational modifications such as glycosylation, transglutamination and cross-linking can all profoundly effect tissue mechanical properties and thus influence stem cell function via mechanotransduction.Citation5 Therefore, understanding how chronic inflammation influences these properties and processes will provide important insight into the molecular factors linking inflammation and mechanotransduction. Because the chronic inflammatory environment is a complex space harboring a variety of cell types and biochemical factors, the underlying mechanisms are likely to be multifaceted and will involve direct and indirect regulation. For example, inflammatory cells may secrete biochemical factors that directly modulate the production, deposition and/or organization of ECM components.Citation6 Alternatively, alteration of the ECM may occur as a secondary consequence following changes in other aspects of the tissue microenvironment, such as changes in metabolic function that accompany inflammatory responses.Citation6,7
In addition to delineating the molecular factors by which inflammation regulates ECM remodeling, higher resolution analysis of how the physical and structural properties of the niche microenvironment change in response to inflammation will also be highly informative when considering how stem cells are controlled by mechanical cues. The use of technologies such as Atomic Force Microscopy will be extremely useful in this regard.
Finally, in addition to understanding how aberrant inflammatory responses contribute to disease, further elucidation of the link between inflammation, ECM remodeling and mechanotransduction will advance our understanding of the normal regenerative response during tissue repair, which will have important implications for regenerative medicine.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Arwert EN, et al. Nat Rev Cancer 2012; 12:170-80; PMID:22362215; http://dx.doi.org/10.1038/nrc3217
- Mariani F, et al. World J Gastroenterol 2014; 20:9716-31; PMID:25110410; http://dx.doi.org/10.3748/wjg.v20.i29.9716
- Hsu YC, et al. Nat Med 2014; 20:847-56; PMID:25100530; http://dx.doi.org/10.1038/nm.3643
- Nowell CS, et al. Nat Cell Biol 2015; PMID:26689676
- Cox TR, Erler JT. Dis Model Mech 2011; 4:165-78; PMID:21324931; http://dx.doi.org/10.1242/dmm.004077
- Karin M, Clevers H. Nature 2016; 529:307-15; PMID:26791721; http://dx.doi.org/10.1038/nature17039
- Rovida E, et al. Cell Cycle 2014; 13:3169-75; PMID:25485495; http://dx.doi.org/10.4161/15384101.2014.964107
