ABSTARCT
Formation of the XY body is believed to prevent recombination between X and Y chromosomes during meiosis. We recently demonstrated that SYCP3-like X-linked 2 (Slx2) could be involved in synaptonemal complex formation as well as XY body maintenance during meiosis. In order to further investigate the role and composition of XY body protein complexes in meiotic processes and spermatogenesis, a yeast 2-hybrid screening was performed, and the tripartite motif protein 27(Trim27) was found to interact with Slx2 and co-localized in the XY body. Trim27 has a tripartite motif (TRIM) consisting of a RING finger, B-box and coiled-coil domains, and is a transcriptional regulator that is expressed in various tumor cell lines. In this study, we showed that Slx2 and Trim27 were highly expressed in meiosis of mouse testis. And the Slx2/Trim27 interaction was confirmed in vivo by co-immunoprecipitation and mammalian 2-hybrid interaction assays. Moreover, cytoimmuno localization experiments revealed that Slx2/Trim27 was co-localized to the XY body of spermatocytes during meiosis, and immunohistochemical results revealed co-localization of Trim27 and γ-H2AX in the XY body of primary spermatocytes in the mouse testis. Trim27 may therefore be a transcriptional regulation protein connecting Slx2 and γ-H2AX, thereby promoting the formation of a more potent XY body protein complex in meiotic processes and spermatogenesis. In conclusion, Trim27 connecting Slx2 may regulate meiotic processes in multiple ways by influencing XY body formation and germ cell proliferation during spermatogenesis.
KEYWORDS:
Introduction
Spermatogenesis is a complex biological process involving both mitotic and meiotic cell division.Citation1 During spermatogenesis, the X and Y chromosomes are transcriptionally silenced during the meiotic prophase and form a condensed chromatin domain known as the XY body.Citation2-5 Formation of the XY body is believed to prevent recombination between X and Y chromosomes and protect them from double stranded breaks (DSBs).Citation2 However, the molecular mechanisms underlying sex chromosome condensation remain known. The recent discovery of specific proteins located within the XY body has aided our understanding of its structure and function.Citation6-9 The phosphorylated form of H2AX (γ-H2AX) globally distributed during the leptotene and zygotene stages, but becomes concentrated at the asynapsed XY body during the pachytene and diplotene stages.Citation10 Moreover, XMR, a member of the X-linked lymphocyte regulated (Xlr) family, is concentrated on a chromosome segment emerging from the XY body.Citation11 We recently demonstrated that SYCP3-like X-linked 2 (Slx2) might be involved in synaptonemal complex formation as well as XY body maintenance during meiosis.Citation9,12 In spermatocytes, Slx2 is distributed in the nucleus of germ cells during the preleptotene, leptotene and zygotene stages, but is restricted to the XY body at the pachytene stage. To date, Slx2 have been found to highly express in the testis and may be involved in homologous recombination, double strand repair and sex chromosomes inactivation during the meiosis.Citation9 However, the role and composition of XY body protein complexes (include Slx2) have not been clearly in testis during the meiosis.
In order to further investigate the role and composition of XY body protein complexes in spermatogenesis and identify other potential proteins that form the XY body, a yeast 2-hybrid screen was performed, and tripartite motif protein 27 (Trim27) was found to interact with Slx2. Trim27 (also known as the Ret finger protein, RFP) is a member of the tripartite motif-containing (TRIM) family and contains a TRIM/RBCC motif that consists of a RING domain, one or 2 B-box domains, and a predicted coiled-coil region.Citation13,14 TRIM proteins form a superfamily with ∼65 members in humans and mice,Citation15 and they are known to play important roles in a wide range of processes including cell growth, tumor suppression, DNA damage signaling, senescence, apoptosis, stem cell differentiation, and immune responses against viral and particularly HIV infection.Citation15-21 Although Trim27 is highly expressed in various tumor types and testis,Citation22 the exact expression pattern, cellular localization and physiological function of Trim27 in testis remain unclear.
Here we showed that Trim27 were highly expressed in the mouse testis, and differentially expressed during spermatogenesis. Moreover, the Slx2 and Trim27 proteins were localized at the nucleus of primary spermatocytes, especially at the XY body during meiosis process. Furthermore, we identified Trim27 as was found to interact with Slx2 using yeast 2-hybrid screening and co-immunoprecipitation (co-IP). In conclusion, Trim27 was co-localized with Slx2 and γ-H2AX in the XY body, suggesting Trim27 connecting Slx2 could play multiple roles in the regulation of XY body formation and germ cell proliferation during spermatogenesis.
Results
Expression of Trim27 and Slx2 mRNA and protein in mouse testes
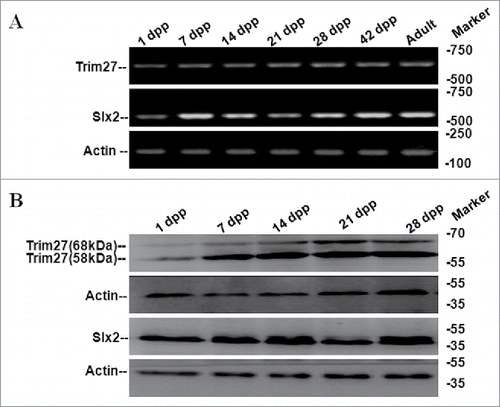
Trim27 and Slx2 expression were investigated in testes during different developmental stages. Trim27/Slx2 mRNA and protein were detected by RT-PCR () and western blotting (), respectively. And Trim27 mRNA (protein) expression pattern matched Slx2 mRNA (Protein) expression in testes.Trim27 mRNA was detected in 1 dpp testis tissue after birth (), and protein expression matched them RNA expression pattern. Trim27 appeared as a 58 kDa testis protein by western blotting, but a unique 68 kDa protein was also detected (), suggesting Trim27 may be present in more than one form. Trim27 protein was not abundant in the 1dpp testis, but levels were higher at 14 dpp in cells undergoing meiotic prophase during the first cycle of spermatogenesis, suggesting Trim27 may be involved in male germ cell development.
Figure 1. Expression of mouse Trim27 and Slx2 detected by RT-PCR and western blotting. (A)Trim27 and Slx2 expression were investigated in multiple developmental stages in mouse testis tissue by RT-PCR, and expression was high in various different stages. (B) Trim27 and Slx2 proteins were detected by Western blotting following immune precipitation. Two distinct bands of Trim27 were detected that correspond to 58 kDa and 68 kDa proteins.
Trim27 was localized to the nucleus and cytoplasm
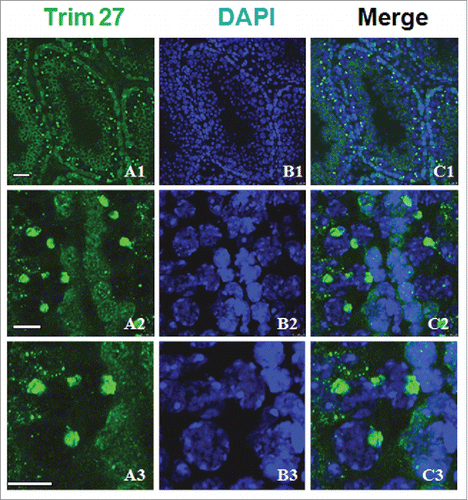
We performed immunostaining of Trim27 on gonadal sections to examine the distribution in different germ cells (). Immunostaining of Trim27 in adult testis using DAPI revealed localization in the nucleus of primary spermatocytes. No specific staining was detected in spermatogenic cells when only type A or B spermatogonia were present. Trim27 was localized in the cytoplasm of round spermatids and the nuclei of Sertoli cells, and therefore appears to be a nuclear protein in spermatocytes () but a cytoplasmic protein in round spermatids, indicating a role in spermatid cell proliferation.
Figure 2. Trim27 expression in germ cells as detected by immunostaining of testis sections. Upper panel: Trim27 (green) and nuclei (blue) at low magnification. Lower panel: Trim27 (green) and nuclei (blue) at high magnification. Trim27 is both nuclear and cytoplasmic, and is localized in the nuclei of primary spermatocytes, but in the cytoplasm of round spermatids. Bars = 10 μm.
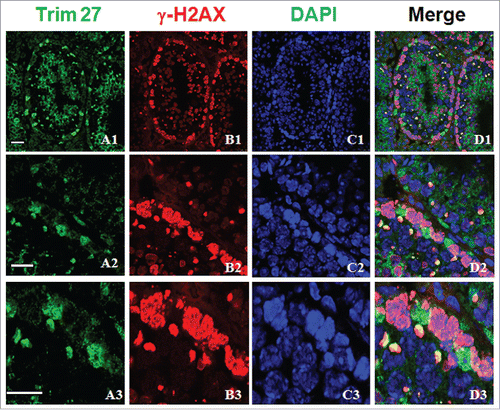
When meiosis reached the pachytene stage, smaller stained spots were observed in some tubules of adult mice, which were assumed to be XY bodies based on their size and morphology. Trim27 and γ-H2AX were co-localized at nuclei during the zygotene stage (), and since γ-H2AX is a well-known marker of the XY body, this confirmed that Trim27 was indeed associated with meiosis. We then directly examined the co-localization of Trim27 with which confirmed that Trim27 was indeed associated with meiosis. Upon further investigation of the co-localization of Trim27 and γ-H2AX in chromosome spreads of spermatocytes, co-localization at nuclei was evident at the zygotene stage, while co-localization at XY bodies was apparent in pachytene spermatocytes ().
Figure 3. Co-localization of Trim27 and γ-H2AX in testis sections. Upper panel: Trim27 (green), γ-H2AX (red) and nuclei(blue) at low magnification. Lower panel: Trim27 (green), γ-H2AX (red) and nuclei (blue) at high magnification. Trim27 is co-localized with γ-H2AX in the nuclei of spermatocytes at the zygotene stage, and in the XY bodies of pachytene spermatocytes. Bars = 10 μm.
Physical interaction between Trim27 and Slx2
In order to define the biochemical role of Slx2 in XY body, a yeast 2-hybrid screening was employed to identify potential binding partners, and Trim27 was identified as a candidate.
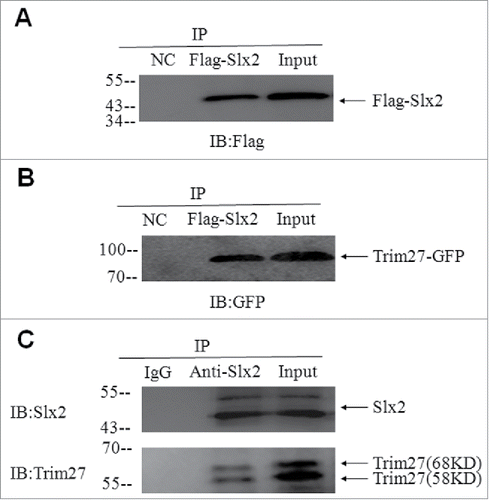
The interaction between Slx2 and Trim27 was initially observed in the yeast 2-hybrid experiment, and the interaction was further confirmed using co-IP on HEK293T cell lysates from cells co-transfected with FLAG-Slx2 and Trim27-GFP plasmids (). Moreover, Trim27-GFP could be pulled down by the GST-Slx2 fusion protein, and even more importantly, the in vivo interaction between Trim27 and Slx2 was confirmed using co-IP on the adult testicular lysates ().
Figure 4. Interaction between Trim27and Slx2, identified using yeast 2-hybrid analysis and confirmed using co-immunoprecipitation (CoIP). (A and B). CoIP using lysates from HEK293T cells co-transfected with Trim27-GFP and FLAG- Slx2. (C). The in vivo interaction between Trim27 and Slx2 was confirmed by Co-IP experiments on testicular lysates.
The Slx2-Trim27 complex was localized at XY bodies
We next examined whether Slx2 and Trim27 co-localized in meiotic spermatocytes. Immunofluorescence staining of the chromosome spreads of spermatocytes using a confocal laser scanning microscope indicated slight localization of Trim27 at bright satellite sequences (). Slx2 and Trim27 were found to co-localize at XY bodies in primary meiotic spermatocytes, but were separated in round spermatids, where Slx2 was restricted to XY bodies while Trim27 was diffuse in the cytoplasm of differentiating sperm.
Figure 5. Immunostaining of testes chromosome spreads at the leptotene and diplotene stages for Trim27/SYCP3 and Slx2/Scp3. At the pachytene and diplotene stages, Trim27 and Slx2 are specifically restricted to XY bodies, and both proteins share a similar distribution pattern at the zygotene and diplotene stages. Interaction between Trim27 and Slx2 appears to occur in XY bodies, suggesting Trim27 is a linker protein that mediates the interaction between Slx2 and XY body proteins, and may therefore control XY body formation during meiosis. Images were captured using a laser confocal microscope. Bar = 5 µm.
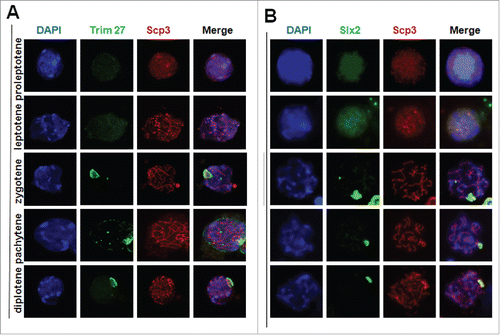
To confirm that Trim27 was indeed localized at XY bodies of SC, chromosome spreads of cells in the leptotene to diplotene stages were immunostained for the Trim27/Scp3 and Slx2/Scp3. In preleptotene spermatocytes, Scp3 was distributed evenly across the entire nucleus, and the expression and distribution of Trim27 was similar to that of Scp3. In zygotene spermatocytes, Scp3 was beginning to be visible whereas Trim27 was diffuse but unevenly distributed. In the pachytene stage, Slx2 and Trim27 were both restricted to XY bodies while Scp3 was localized specifically at the SC. During the diplotene stage, Slx2 and Trim27 remained localized at XY bodies where as Scp3 at the SC had begun to disassemble. In summary, Slx2 and Trim27 shared a similar distribution pattern at the zygotene and diplotene stages of meiosis, and the interaction between Slx2 and Trim27 appeared to occur in XY bodies. The results suggested Trim27 connecting Slx2 could play important roles in the XY body formation during meiosis.
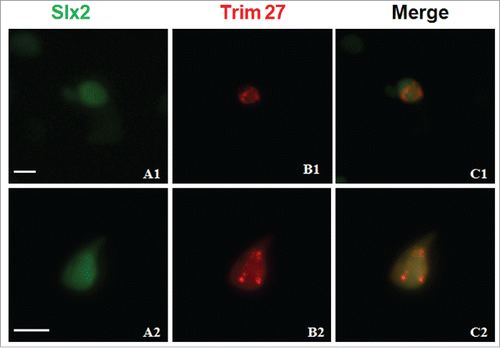
To examine the interaction and localization between Trim27 and Slx2 in vivo, Slx2-GFP and Trim27-RFP fusion proteins were expressed in HEK293T cells (). Trim27-RFP was manly localized as speckles in the nuclei, and Slx2-GFP was also displayed a nuclear localization but speckles were not evident. Interaction between Trim27 and Slx2 may be essential for XY body formation in the nuclei of primary spermatocytes.
Figure 6. Subcellular localization of Trim27 and its influence on the distribution of Slx2 in HEK293T cells. Slx2-GFP is co-localized with Trim27-RFP in the nuclei of cells co-transfected with Slx2-GFP and Trim27-RFP fusion plasmids. The nuclei of cultured HEK293T cells are stained with DAPI, and images were captured using a laser confocal microscope. Upper panel: Trim27 (red), Slx2 (green) and nuclei (blue) at low magnification. Lower panel: Trim27 (red), Slx2 (green) and nuclei (blue) at high magnification. Bars = 10 μm.
Discussion
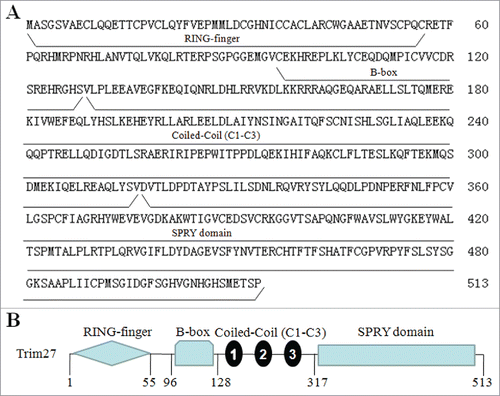
Formation of the SC during the meiotic stage of spermatogenesis is a key event. During prophase of the meiotic process, X and Y chromatin is remodelled to form a specialized, morphologically distinct subnuclear domain known as the XY body,Citation7 the formation of which may protect the X and Y chromosomes from damaging recombination events that could occur because they are not completely homologous.Citation2,7 Previously, we identified the novel XY body component protein Slx2, which is expressed in meiotic germ cells and is localized at XY bodies.Citation9,12 In the present study, we found that Trim27 was highly expressed in mouse testis and localized at the same cellular substructures. The full-length Trim27 cDNA is predicted to encode a protein of 513 amino acids (), and mouse Trim27 shares 98.4% homology with the human sequence. Trim27 was first identified by DNA rearrangementCitation23 and mapped to mouse chromosome 13. Trim27 is a member of the TRIM family of proteins, and consists of a RING domain, a B-box domain, 3 coiled-coil regions and one SPLA kinase andryanodine receptor (SPRY) domain (). The RING domain plays a critical role in mediating the transfer of ubiquitin, and is a characteristic signature of many E3 ubiquitin ligases. The B-box domain includes a zinc-binding motif and is a critical determinant of the tripartite motif. The coiled coil region in TRIM/RBCC proteins is mainly involved in homo-oligomerization, promoting the formation of high molecular weight complexes, and defining discrete subcellular compartments within the cell.Citation24 E3 activity is dependent on the TRIM motif. The SRRY domain of the C-terminal region () has no known function in any of the TRIM/RBCC proteins characterized to date.Citation25 Interestingly, a major band (∼68 KDa) And the expression and precise underlying mechanisms of Slx2 should be clearly research in testis. a minor one (∼58 KDa ) were observed on the western blots of the mouse testis (). To confirm that the proteins detected by the antibody were indeed isoforms of Trim27, we concentrated the proteins from testicular lysate by immunoprecipiation (IP) using the same polyclonal antibody as used in protein gel blotting, collected the proteins from the Comassie Blue-stained gels, and subjected the samples to mass spectrometry analysis. The results should that both isoforms contained Trim27 peptides (data not shown). The presence of the 68kd isoform was not affected by kinase treatment or preparation of extracts in the absence of protease inhibitors suggesting that modification is not phosphorylation, ubiqitination or sumoylation.
Figure 7. Amino acidsequence and structural domains of Trim27. (A)The full-length Trim27 cDNA is predicted to encode a protein of 513 amino acids; (B) Trim27 is a member of the TRIM family, and consists of a RING domain, a B-box domain, 3 coiled-coil regions (C1 to C3) and an SPRY domain.
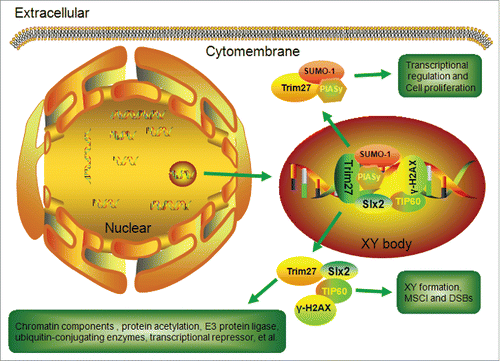
A more detailed surface spread analysis showed that Trim27 was localized at XY bodies. Interestingly, Sumo-1 is also localized at XY bodies in pachytene spermatocytes.Citation7,8 Recent results show that Trim27, Sumo-1 and PIAS all localize at a characteristic nuclear structure that is juxtaposed with the inner nuclear membrane (XY body) of primary spermatocytes in mouse testis.Citation26 Moreover, Trim27 was suggested to have an important role in the enhancement of transcriptional repression in spermatogenesis, and MBD proteins appear to be involved.Citation27 In addition, Trim27 interacts with enhancer of polycomb1 (EPC1) and functions as a transcriptional repressor in cultured cells.Citation28 The distribution of Trim27 in meiotic spermatocytes implies that it is a mediator that connects Slx2, Sumo-1 and MBD proteins to facilitate formation of the XY body during meiosis (). We hypothesize that Trim27 connecting Slx2 acts as an XY body component crucial for XY body formation and transcriptional regulation during meiosis.
Meiotic sex chromosome inactivation (MSCI) occurs during the meiotic phase of spermatogenesis, and involves transcriptional silencing of the X and Y chromosomes. MSCI has been shown to contribute to formation of the XY body.Citation29 It is initiated by several DNA repair proteins, and is maintained through histone modifications or other modifications during the meiotic stage. At the leptotene and zygotene substages, the X and Y chromosomes are still transcriptionally active. However, they are rapidly silenced shortly after the zygotene-to-pachytene transition, which is when XY bodies are formed. Until recently, the transcriptional status of the X and Y chromosomes during spermatogenesis was poorly understood, because few published studies have focused on the activity of specific X- or Y-linked genes. H2AX performs an essential role in MSCI, and γ-H2AX is abundant in the mouse testis and is localized at XY bodies.Citation30 γ-H2AX plays an important role in the response to DNA damage such as DSBs during the leptotene stage.Citation31 γ-H2AX is phosphorylated at the site of DSBs, which in turn recruits DNA repair factors that participate in DSB repair following homogenous recombinant.Citation32 In the present study, we found Trim27 and γ-H2AX to be co-localized at XY bodies during the meiotic stage. The involvement of γ-H2AX in XY body formation has been associated with sex inactivation,Citation33 and Trim27 may mediate γ-H2AX in this process. Previously, we suggested that the interaction between Slx2and TIP60 could be involved in DNA recombination, SC formation and XY body maintenance during meiosis.Citation9 In addition to interacting with Slx2, Trim27 is known to interact with Structural Maintenance of Chromosome 3 (SMC3), a component of the multimeric cohesion complex that holds sister chromatids together and prevents their premature separation during mitosis.Citation34 It is possible that Trim27 may recruit γ-H2AX and Slx2 and/or SMC3 during DSB repair and MSCI during meiosis ().Given the spatial distribution of Trim27 in spermatogenesis, we hypothesize that Trim27 connecting Slx2 acts as a novel XY body component associated with MSCI during meiosis in spermatocytes.
The prototype member of the X-linked lymphocyte regulated (XLR) protein super family has been linked to X-linked immunodeficiency in mice. XLR proteins contain a COR1 domain, and various family members including Slxl1,Citation1 Slx2,Citation9 Scp3Citation35 and Xlr5cCitation36 play an important role in meiosis during spermatogenesis. Scp3 is an important member of this family that acts as a fundamental component of the lateral elements of the synaptonemal complex during meiosis.Citation37 Along with others, Slx2 expression and interaction with Syce2 and KAT/TIP60 could contribute to meiosis and sex chromosome inactivation.Citation9 And a paper showed that Slx2 KO mice are fertile and have normal meiosis progress by the restricted selection of phenotypic traits.Citation38 So the expression and precise underlying mechanisms of Slx2 were not clearly research in testis. In the present study, closer inspection showed that Trim27 was localized in the cytoplasm of round spermatids, indicating a role in germ cell proliferation. However, Trim27 was localized at XY bodies during different stages of the meiotic cycle, and interacted with Slx2, suggesting an involvement in meiotic progression (). In addition, other evidence indicates that Trim27 may be associated with other subcellular processes and chromatin components,Citation33 ubiquitin-conjugating enzymes Citation39,40 the E3 sumo protein ligase PIAS Citation26 and protein acetylation,Citation41 and may also inhibit activation of gene transcription.Citation42,43 Our findings suggest that Trim27 and Slx2 may be cooperatively involved in normal spermatogenesis. It was recently reported that PIAS acts as a Sumo E3 ligases for Trim27, and the association of PIAS and Trim27 may be important for transcriptional repression in spermatogenesis.Citation26 Our results suggest that Trim27 acts as a transcriptional regulation protein that, along with Slx2, functions in the transcriptional regulation of XY body formation and germ cell proliferation during spermatogenesis.
In summary, we revealed an interaction between Trim27 and Slx2, and the localization of this complex at XY bodies during meiosis in spermatogenesis. The abundant expression of Trim27 and Slx2 in germ cells and localization at XY bodies suggests Trim27/Slx2 interaction may perform multiple roles in the regulation of spermatogenesis including XY body formation, MSCI and germ cell proliferation.
Materials and methods
Ethics statement
This work was approved by the Institutional Animal Care and Use Committee (IACUC) of the Peking University Third Hospital (Protocol Number: 2013SZ021). All animal experiments conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals, issued by the Peking University Third Hospital in Beijing.
Animals and reagents
All animals were obtained from the Experimental Animal Center, Peking University Health Science Center. The Institute Committee on Animal Care and Use approved all protocols for animal treatment.
Reagents for cell culturing and animal treatment were obtained from Sigma (St. Louis, MO, USA), Invitrogen (Carlsbad, CA, USA) and Beijing Chemical Reagents (Beijing, China) unless specified otherwise. Protein Agarose and anti-GFP purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).Mouse polyclonal anti-SYCP3 antibody was obtained from Novus Biologicals (Litteleton, CO, USA). Mouse monoclonal γ-H2AX and anti-Trim27 antibodies were obtained from Abcam (Cambridge, UK).
Preparation of RNA and RT-PCR
Total RNA in testes at different stages of development was extracted for RT-PCR analysis using Trizol reagent (Invitrogen, 15596018, USA) as described in our previous study.Citation44 Total RNA (5 μg) was used as template for reverse transcription using Superscript III (Invitrogen) according to the manufacturer's protocol. The Matchmaker library construction and screening kit was purchased from Clontech (BD Biosciences. San Jose, CA, USA). Trim27 primer pairs (5′-ACT CTCGAGATGACCCAGGACTGTGCAGAG-3′and 5′-ACAGGTACCTCAGATTAC AGGCAACAGCACTT-3′) were used to quantify Trim27 mRNA expression, and the resulting PCR products were subcloned into the pGEX-4T1 vector and sequenced to ensure there were no mutations due to PCR errors. The products of RT-PCR and PCR were analyzed by electrophoresis and stained with ethidium bromide. GAPDH was included as an internal positive control of the amplification and integrity of extracts.
Western blotting
Western blotting was performed using a standard protocol. Briefly, equal amounts of testis protein (20∼50 μg total protein/lane) were separated by 10% SDS-PAGE, transferred to nitrocellulose membranes (Amersham Biosciences AB, Uppsala, Sweden), blocked in TBST (0.5% Tween-20 in TBS) containing 5% non-fat milk powder for 1 h, incubated with primary antibody followed by incubation with HRP-conjugated secondary antibody. Proteins were visualized using an Enhanced Chemiluminescence Kit (Pierce. Rockford, IL, USA), and protein expression levels were determined using Image-Pro plus software 5.1.
Yeast two-hybrid screening
Yeast two-hybrid screening was performed using the Matchmaker library construction and screening kit with slight modifications.Citation12 The bait plasmid was constructed by subcloning the full-length mouse Trim27 cDNA into pGBKT7. Fusion library construction and 2-hybrid assays were carried out in one step by co-transforming the yeast strain AH109 with dscDNA, pGADT7-Rec and pGBKT7. After selection on SD-Ade/-His/-Leu/-Trp plates, cDNA encoding the interacting polypeptides was isolated from positive colonies and sequenced. Inserts from selected positive clones were sequenced and searched against the NCBI database using BLAST.
Antibodies and immunoblotting
Immunostaining of frozen mouse testicular tissue was performed as previously described.Citation36 Frozen sections (6∼8 µm) of mouse testis were fixed immediately in 4% paraformaldehyde for 15 min at room temperature. After washing, sections were blocked with 1% BSA in PBS at room temperature for 1 h. After blocking, sections were incubated with Trim27 antibody (diluted 1:300 in blocking buffer) or pre-immune rabbit serum (negative control) for 1 h at room temperature. Incubation with the FITC-conjugated anti-rabbit antibody (1:500) (Jackson Laboratories, Cambridgeshire, UK) was then followed by incubation with DAPI (Sigma–Aldrich).
Chromosome spreading of primary spermatocytes was performed as previously described.Citation9 Slides were stained using anti-Scp3 (1:200, Novus Biologicals), anti-γH2AX (1:200, Abcam), and anti-Trim27 (1:300 Abcam). TRITC-conjugated or FITC-conjugated anti-rabbit antibodies (1:500) (Jackson Laboratories, Cambridgeshire, UK) were then applied, followed by incubation with DAPI.
Co-immunoprecipitation and confocal microscopy
Trim27 and Slx2 gene fragments were subcloned into pFlag-cmv4 and pEGFP-N1 vectors to construct the Flag-Slx2 and Trim27-GFP tagged plasmids, which were subsequently co-transfected into HEK293T cells. Total protein cell lysates were incubated with rabbit immunoglobulin (IgG) or anti-GFP antibody for 2 h at 4°C, followed by incubation with protein A agarose (Santa Cruz) overnight at 4°C. Agarose beads and captured protein complexes were washed 6 times and suspended in SDS sample buffer for immunoblotting with anti-Flag (1:2000 dilution, Sigma–Aldrich), anti-GFP (1:1000 dilution, Santa Cruz) or anti- Slx2 (1:3000 dilution).
GST pull-down assays
A full-length Slx2 cDNA fragment was subcloned into the pGEX-4T1 vector to construct the GST-Slx2 tagged plasmid. GST and GST-Slx2 were produced in Escherichia coli BL21 and purified using a GSTrap FF column (Amersham Biosciences). For the GST pull-down assay, GST and GST- Slx2 fusion proteins were immobilized on glutathione sepharose beads by incubating for 1 hour at 4°C, and beads were subsequently washed 4 times with TEN100 buffer (20 mM Tris–HCl, 1 mM EDTA, 100 mM NaCl, pH 7.4). Trim27-GFP fusion protein was over-expressed in HEK293T cells, and total protein cell lysates were extracted with lysis buffer (150 mM NaCl, 50 mM Tris–HCl pH 8.0, 0.5% Nonidet-P40) containing protease inhibitor cocktail (Roche)and incubated with beads for 2 h at 4°C. Beads were washed 6 times with lysis buffer then suspended in SDS sample buffer. Protein lysates were separated by SDS-PAGE and immunoblotted with anti-Slx2 or GFP antibody.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Prof. Chunsheng Han and Dr. Yu-Qiang Shi for the valuable recommendation and technical assistance.
Funding
This study was supported by the National Natural Science Foundation of China for Young Scholars (NO. 81671513, 81200466 and 31401256).
References
- Zhuang XJ, Hou XJ, Liao SY, Wang XX, Cooke HJ, Zhang M, Han C. SLXL1, a novel acrosomal protein, interacts with DKKL1 and is involved in fertilization in mice. PLoS One 2011; 6:e20866; PMID:21698294; http://dx.doi.org/10.1371/journal.pone.0020866
- McKee BD, Handel MA. Sex chromosomes, recombination, and chromatin conformation. Chromosoma 1993; 102:71-80; PMID:8432196; http://dx.doi.org/10.1007/BF00356023
- Solari AJ. The behavior of the XY pair in mammals. Int Rev Cytol 1974; 38:273-317; PMID:4854664; http://dx.doi.org/10.1016/S0074-7696(08)60928-6
- Nora EP, Heard E. Chromatin structure and nuclear organization dynamics during X-chromosome inactivation. Cold Spring Harb Symp Quant Biol 2010; 75:333-44; PMID:21447823; http://dx.doi.org/10.1101/sqb.2010.75.032
- Kamikawa Y, Donohoe ME. The dynamics of X-chromosome inactivation in mouse development. Mol Reprod Dev 2014; 81:141-7; PMID:24243482; http://dx.doi.org/10.1002/mrd.22282
- Hoyer-Fender S. Molecular aspects of XY body formation. Cytogenet Genome Res 2003; 103:245-55; PMID:15051945; http://dx.doi.org/10.1159/000076810
- Handel MA. The XY body: a specialized meiotic chromatin domain. Exp Cell Res 2004; 296:57-63; PMID:15120994; http://dx.doi.org/10.1016/j.yexcr.2004.03.008
- Rogers RS, Inselman A, Handel MA, Matunis MJ. SUMO modified proteins localize to the XY body of pachytene spermatocytes. Chromosoma 2004; 113:233-43; PMID:15349788; http://dx.doi.org/10.1007/s00412-004-0311-7
- Shi YQ, Zhuang XJ, Xu B, Hua J, Liao SY, Shi Q, Cooke HJ, Han C. SYCP3-like X-linked 2 is expressed in meiotic germ cells and interacts with synaptonemal complex central element protein 2 and histone acetyltransferase TIP60. Gene 2013; 527:352-9; PMID:23810942; http://dx.doi.org/10.1016/j.gene.2013.06.033
- Fernandez-Capetillo O, Liebe B, Scherthan H, Nussenzweig A. H2AX regulates meiotic telomere clustering. J Cell Biol 2003; 163:15-20; PMID:14530383; http://dx.doi.org/10.1083/jcb.200305124
- Escalier D, Garchon HJ. XMR is associated with the asynapsed segments of sex chromosomes in the XY body of mouse primary spermatocytes. Chromosoma 2000; 109:259-65; PMID:10968254; http://dx.doi.org/10.1007/s004120000075
- Zhuang XJ, Shi YQ, Xu B, Chen L, Tang WH, Huang J, Lian Y, Liu P, Qiao J. SLX2 interacting with BLOS2 is differentially expressed during mouse oocyte meiotic maturation. Cell Cycle 2014; 13:2231-7; PMID:24870619; http://dx.doi.org/10.4161/cc.29265
- Tezel GG, Ordulu Z, Himmetoglu C, Usubutun A. The selective expression of ret finger protein in endometrial cancer: can RFP be a marker of serous carcinomas? Turk Patoloji Derg 2012; 28:213-9; PMID:23011823
- Freemont PS. The RING finger. A novel protein sequence motif related to the zinc finger. Ann N Y Acad Sci 1993; 684:174-92; PMID:8317827; http://dx.doi.org/10.1111/j.1749-6632.1993.tb32280.x
- Ozato K, Shin DM, Chang TH, Morse HC, 3rd. TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol 2008; 8:849-60; PMID:18836477; http://dx.doi.org/10.1038/nri2413
- Schwamborn JC, Berezikov E, Knoblich JA. The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell 2009; 136:913-25; PMID:19269368; http://dx.doi.org/10.1016/j.cell.2008.12.024
- Tomar D, Singh R. TRIM family proteins: emerging class of RING E3 ligases as regulator of NF-kappaB pathway. Biol Cell 2015; 107:22-40; PMID:25319221; http://dx.doi.org/10.1111/boc.201400046
- Liu C, Huang X, Hou S, Hu B, Li H. Silencing of tripartite motif (TRIM) 29 inhibits proliferation and invasion and increases chemosensitivity to cisplatin in human lung squamous cancer NCI-H520 cells. Thorac Cancer 2015; 6:31-7; PMID:26273332; http://dx.doi.org/10.1111/1759-7714.12130
- Tabah AA, Tardif K, Mansky LM. Anti-HIV-1 activity of Trim 37. J Gen Virol 2014; 95:960-7; PMID:24317724; http://dx.doi.org/10.1099/vir.0.057653-0
- Cambiaghi V, Giuliani V, Lombardi S, Marinelli C, Toffalorio F, Pelicci PG. TRIM proteins in cancer. Adv Exp Med Biol 2012; 770:77-91; PMID:23631001; http://dx.doi.org/10.1007/978-1-4614-5398-7_6
- Nisole S, Stoye JP, Saib A. TRIM family proteins: retroviral restriction and antiviral defence. Nat Rev Microbiol 2005; 3:799-808; PMID:16175175; http://dx.doi.org/10.1038/nrmicro1248
- Cao T, Shannon M, Handel MA, Etkin LD. Mouse ret finger protein (rfp) proto-oncogene is expressed at specific stages of mouse spermatogenesis. Dev Genet 1996; 19:309-20; PMID:9023983; http://dx.doi.org/10.1002/(SICI)1520-6408(1996)19:4%3c309::AID-DVG4%3e3.0.CO;2-D
- Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 1985; 42:581-8; PMID:2992805; http://dx.doi.org/10.1016/0092-8674(85)90115-1
- Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, et al. The tripartite motif family identifies cell compartments. EMBO J 2001; 20:2140-51; PMID:11331580; http://dx.doi.org/10.1093/emboj/20.9.2140
- Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays 2005; 27:1147-57; PMID:16237670; http://dx.doi.org/10.1002/bies.20304
- Matsuura T, Shimono Y, Kawai K, Murakami H, Urano T, Niwa Y, Goto H, Takahashi M. PIAS proteins are involved in the SUMO-1 modification, intracellular translocation and transcriptional repressive activity of RET finger protein. Exp Cell Res 2005; 308:65-77; PMID:15907835; http://dx.doi.org/10.1016/j.yexcr.2005.04.022
- Fukushige S, Kondo E, Gu Z, Suzuki H, Horii A. RET finger protein enhances MBD2- and MBD4-dependent transcriptional repression. Biochem Biophys Res Commun 2006; 351:85-92; PMID:17049487; http://dx.doi.org/10.1016/j.bbrc.2006.10.005
- Tezel G, Shimono Y, Murakumo Y, Kawai K, Fukuda T, Iwahashi N, Takahashi M. Role for O-glycosylation of RFP in the interaction with enhancer of polycomb. Biochem Biophys Res Commun 2002; 290:409-14; PMID:11779184; http://dx.doi.org/10.1006/bbrc.2001.6161
- Takada Y, Isono K, Shinga J, Turner JM, Kitamura H, Ohara O, Watanabe G, Singh PB, Kamijo T, Jenuwein T, et al. Mammalian Polycomb Scmh1 mediates exclusion of Polycomb complexes from the XY body in the pachytene spermatocytes. Development 2007; 134:579-90; PMID:17215307; http://dx.doi.org/10.1242/dev.02747
- Celeste A, Difilippantonio S, Difilippantonio MJ, Fernandez-Capetillo O, Pilch DR, Sedelnikova OA, Eckhaus M, Ried T, Bonner WM, Nussenzweig A. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell 2003; 114:371-83; PMID:12914701; http://dx.doi.org/10.1016/S0092-8674(03)00567-1
- Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet 2001; 27:271-6; PMID:11242108; http://dx.doi.org/10.1038/85830
- Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, et al. Genomic instability in mice lacking histone H2AX. Science 2002; 296:922-7; PMID:11934988; http://dx.doi.org/10.1126/science.1069398
- Vigodner M. Sumoylation precedes accumulation of phosphorylated H2AX on sex chromosomes during their meiotic inactivation. Chromosome Res 2009; 17:37-45; PMID:19156530; http://dx.doi.org/10.1007/s10577-008-9006-x
- Patel CA, Ghiselli G. The RET finger protein interacts with the hinge region of SMC3. Biochem Biophys Res Commun 2005; 330:333-40; PMID:15781269; http://dx.doi.org/10.1016/j.bbrc.2005.02.162
- Yuan L, Liu JG, Zhao J, Brundell E, Daneholt B, Hoog C. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell 2000; 5:73-83; PMID:10678170; http://dx.doi.org/10.1016/S1097-2765(00)80404-9
- Zhuang XJ, Tang WH, Liu CY, Zhu JL, Feng X, Yan J, Lian Y, Liu P, Qiao J. Identification and Characterization of Xlr5c as a Novel Nuclear Localization Protein in Mouse Germ Cells. PLoS One 2015; 10:e0130087; PMID:26075718; http://dx.doi.org/10.1371/journal.pone.0130087
- Scherthan H, Weich S, Schwegler H, Heyting C, Harle M, Cremer T. Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J Cell Biol 1996; 134:1109-25; PMID:8794855; http://dx.doi.org/10.1083/jcb.134.5.1109
- Li M, Huang R, Jiang X, Chen Y, Zhang Z, Zhang X, Liang P, Zhan S, Cao S, Songyang Z, et al. CRISPR/Cas9 Promotes Functional Study of Testis Specific X-Linked Gene In Vivo. PLoS One 2015; 10:e0143148; PMID:26599493; http://dx.doi.org/10.1371/journal.pone.0143148
- Zheng Q, Hou J, Zhou Y, Yang Y, Xie B, Cao X. Siglec1 suppresses antiviral innate immune response by inducing TBK1 degradation via the ubiquitin ligase TRIM27. Cell Res 2015; 25:1121-36; PMID:26358190; http://dx.doi.org/10.1038/cr.2015.108
- Zaman MM, Nomura T, Takagi T, Okamura T, Jin W, Shinagawa T, Tanaka Y, Ishii S. Ubiquitination-deubiquitination by the TRIM27-USP7 complex regulates tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol 2013; 33:4971-84; PMID:24144979; http://dx.doi.org/10.1128/MCB.00465-13
- Krutzfeldt M, Ellis M, Weekes DB, Bull JJ, Eilers M, Vivanco MD, Sellers WR, Mittnacht S. Selective ablation of retinoblastoma protein function by the RET finger protein. Mol Cell 2005; 18:213-24; PMID:15837424; http://dx.doi.org/10.1016/j.molcel.2005.03.009
- Zha J, Han KJ, Xu LG, He W, Zhou Q, Chen D, Zhai Z, Shu HB. The Ret finger protein inhibits signaling mediated by the noncanonical and canonical IkappaB kinase family members. J Immunol 2006; 176:1072-80; PMID:16393995; http://dx.doi.org/10.4049/jimmunol.176.2.1072
- Bloor AJ, Kotsopoulou E, Hayward P, Champion BR, Green AR. RFP represses transcriptional activation by bHLH transcription factors. Oncogene 2005; 24:6729-36; PMID:16007160; http://dx.doi.org/10.1038/sj.onc.1208828
- Shi YQ, Liao SY, Zhuang XJ, Han CS. Mouse Fem1b interacts with and induces ubiquitin-mediated degradation of Ankrd37. Gene 2011; 485:153-9; PMID:21723927; http://dx.doi.org/10.1016/j.gene.2011.06.025