ABSTRACT
BRAF mutations are known drivers of melanoma development and, recently, were also described as players in the Warburg effect, while this reprogramming of energy metabolism has been identified as a possible strategy for treating melanoma patients. Therefore, the aim of this work was to evaluate the expression and prognostic value of a panel of glycolytic metabolism-related proteins in a series of melanomas. The immunohistochemical expression of MCT1, MCT4, GLUT1, and CAIX was evaluated in 356 patients presenting melanoma and 20 patients presenting benign nevi. Samples included 20 benign nevi, 282 primary melanomas, 117 lymph node and 54 distant metastases samples. BRAF mutation was observed in 29/92 (31.5%) melanoma patients and 17/20 (85%) benign nevi samples. NRAS mutation was observed in 4/36 (11.1%) melanoma patients and 1/19 (5.3%) benign nevi samples. MCT4 and GLUT1 expression was significantly increased in metastatic samples, and MCT1, MCT4 and GLUT1 were significantly associated with poor prognostic variables. Importantly, MCT1 and MCT4 were associated with shorter overall survival. In conclusion, the present study brings new insights on metabolic aspects of melanoma, paving the way for the development of new-targeted therapies.
Introduction
Most solid tumors reprogram their energetic metabolism toward glycolysis, independently from oxygen levels, a phenomenon known as the Warburg effect or aerobic glycolysis.Citation1 Importantly, this metabolic reprogramming was recently identified as one of the hallmarks of cancer Citation2 and has been pointed out as a promising target for anti-cancer therapy.Citation3 Aerobic glycolysis results in a high production of free protons and lactate that must be shuttled to the extracellular milieu through many transporters.Citation4 The resultant microenvironment selects for cells with enhanced metastatic potential and is associated with evasion to immune destruction and resistance to radio- and chemotherapy.Citation5 In this context, monocarboxylate transporters (MCTs), specifically MCT1 and MCT4, play an essential role by contributing both to the hyperglycolytic and the acid-resistant phenotype of cancer cells, by mediating lactate and proton efflux to the extracellular milieu.Citation4 Accordingly, these transporters have been described as upregulated and associated with poor prognosis in many cancer types, with a high potential for exploitation as therapeutic targets.Citation6
Melanoma is the most aggressive skin cancer, with an increasing incidence in the world.Citation7 In the last years, melanoma patient's handling have been changing thanks to an increase in the understanding of melanoma molecular heterogeneity.Citation8 In particular, studies have identified BRAF mutations in these tumors, being mutations associated with an increase in the mortality rate of melanoma patients Citation9 and important for therapeutic decisions thanks to specific inhibitors targeting the signaling pathways involved.Citation10 Specifically, the V600E BRAF mutation is present in 40 to 60% of cutaneous melanoma and accounts for more than 80% of all BRAF mutations,Citation11-13 while NRAS mutations are found in 15 to 20% of melanomas.Citation13,14 The alterations in the MAP kinase cascade due to mutations have been targeted with clinical relevance. Treatment of V600E metastatic melanoma patients with BRAF inhibitors and, more recently, in association with MEK inhibitors, increases patient's survival rates and is approved and available for clinical use.Citation15-18
Interestingly, the V600E BRAF mutation was recently shown to drive the Warburg effect.Citation19 In fact, previous studies in both melanoma and thyroid cancer have shown that this mutation increases the expression of hypoxia-inducible factor-1α (HIF-1α),Citation20,21 the major driver of the Warburg effect in cancer cells.Citation22 Additionally, although mainly studied in the context of tumors different than melanoma, mutations in RAS family members, specifically KRAS, were shown to be associated with a glycolytic phenotype, with increased glucose uptake and lactate production.Citation23-26 Meanwhile, additional studies, both in human samples and in vitro models, have shown that melanoma cells exhibit the Warburg effect Citation27,28 and that the progression to an invasive phenotype occurs under a metabolic switch from mitochondrial oxidative phosphorylation to glycolytic flux followed by lactate production.Citation29 As a result, melanoma cell metabolism has been pointed out as a promising strategy for melanoma treatment.Citation29-31
Although the interest in the metabolic reprogramming of cancer cells is arising in the last years,Citation2 few studies focus on the metabolic profile of melanoma cells. Additional studies should especially evaluate the expression and clinical significance of the metabolism-related proteins, especially those that sustain the Warburg effect. Therefore, the aim of this study was to evaluate the expression and prognostic value of monocarboxylate transporters isoforms 1 and 4 (MCT1, MCT4), glucose transporter 1 (GLUT1) and the pH regulator carbonic anhydrase IX (CAIX), in a series of melanocytic samples including benign nevi, primary tumors and both lymph node and distant metastasis.
Results
Expression of MCTs, GLUT1 and CAIX in melanocytic samples
In benign nevi, with the exception of MCT1 expression that was observed in the plasma membrane, protein expression was almost exclusively found in the cytoplasm. In opposition, in malignant samples, MCT1, MCT4 and GLUT1 expression was almost exclusively found in the plasma membrane (). CAIX exhibited cytoplasmic expression, alone or in combination with plasma membrane expression, however, with plasma membrane predominance. Therefore, and in accordance to the activity of the proteins herein studied, from now on, all results shown are based on plasma membrane expression.
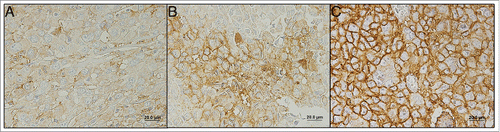
Figure 1. Immunohistochemical expression of MCT1 (A), MCT4 (B), GLUT1 (C) and CAIX (D) in melanoma. All the proteins were more importantly found in the plasma membrane of cells. (A) Stage IIC primary tumor; (B) Stage IIB primary tumor; (C) Distant metastasis; (D) Stage IV primary tumor.
Comparison of protein expression among samples from different origins (benign nevi, melanoma primary tumors, lymph node metastases and distant metastases) showed a significant difference in the overall expression frequency of MCT4, GLUT1 and CAIX (p = 0.030, p = 0.015 and p = 0.009 respectively), and no significant difference for MCT1 (). In the case of MCT4 and GLUT1, the differences observed were due to a significant increase in the transition from primary tumor to lymph-node metastasis, while, for CAIX, a significant increase in the expression frequency was observed in both the transition from benign nevi to primary tumor and lymph-node metastasis to distant metastases. Additionally, considering protein expression in all malignant samples, both MCT1 and MCT4 were significantly co-expressed with GLUT1 and CAIX ().
Table 1. Expression frequencies of MCT1, MCT4, GLUT1 and CAIX in benign nevi, melanoma primary tumors, lymph node metastases and distant metastases. Only plasma membrane expression was considered.
Table 2. Co-expression of MCT1 and MCT4 with GLUT1 and CAIX, in melanoma samples (primary tumors, lymph node metastases and distant metastases). Only plasma membrane expression was considered.
From the 92 patients with results for BRAF mutation status, 29 (31.5%) patients showed the V600E mutation in at least one tumor sample (primary tumor, lymph node metastasis or distant metastasis), while 17 of the 20 benign nevi (85%) showed the V600E mutation. Importantly, MCT1 and GLUT1 were significantly more expressed in BRAF mutated melanoma patients [17/28 (60.7%) and 9/29 (31.0%), respectively] than in BRAF wild-type melanoma patients [23/61 (37.7%) and 8/63 (12.7%), p = 0.043 and p = 0.035, respectively].
From the 36 patients with results for NRAS mutation status, 4 (11.1%) patients showed mutation (Q61H, Q61R, Q61L and G12A) in at least one tumor sample (primary tumor, lymph node metastasis or distant metastasis), while 1 of 19 benign nevi (5.3%) showed the Q61R NRAS mutation. No significant associations were found between NRAS mutation status and expression of the proteins herein analyzed.
Clinicopathological significance of MCTs, GLUT1 and CAIX
The clinicopathological significance of MCTs, GLUT1 and CAIX was analyzed considering plasma membrane expression in primary tumor samples ( and ). MCT1 expression frequency was significantly associated with higher clinical stage (p < 0.001), higher pT (p = 0.016), higher pN (p = 0.004), and higher pM (p = 0.012). MCT4 expression frequency showed the highest number of significant results, being associated with male gender (p = 0.045), higher clinical stage (p = 0.032), nodular histological type (p = 0.029), trunk tumor location (p = 0.038), higher pT (p = 0.002), higher pN (p = 0.008), presence of locoregional recurrence (p = 0.002), higher Breslow's thickness (p = 0.001), and higher number of mitoses/mm2 (p = 0.015). GLUT1 frequency of expression was significantly associated with higher pT (p = 0.035), higher Breslow's thickness (p = 0.036), and higher number of mitoses/mm2 (p = 0.049), while CAIX showed no significant associations with the clinicopathological data.
Table 3. Association of MCT1, MCT4, GLUT1 and CAIX plasma membrane expression with the clinicopathological parameters (categorical variables) in melanomas. Only expression in primary tumors was considered.
Table 4. Association of MCT1, MCT4, GLUT1 and CAIX plasma membrane expression with the clinicopathological parameters (quantitative variables) in melanomas. Only expression in primary tumors was considered.
Survival analysis
The influence of MCTs, GLUT1 and CAIX on overall survival was analyzed considering plasma membrane expression in primary tumor samples ( and ). Overall survival analysis using Kaplan-Meier () showed that MCT1 and MCT4 expression is significantly associated with shorter overall survival (p = 0.037 and p = 0.001, respectively). No significant results were observed for GLUT1 and CAIX expression (data not shown). The predictive prognostic values of the metabolism-related proteins were analyzed by means of Cox proportional hazards regression models (). Univariate analysis showed similar results to the ones obtained with Kaplan-Meier analysis, with significant values for MCT1 and MCT4 expression. However, when applying multivariate analysis, none of these proteins showed to be a stage-independent predictor of overall survival.
Figure 2. Overall survival curves of melanoma's patients. The results are stratified according to protein immunohistochemical expression, using Kaplan Meier's method. Only significant results are shown. Continuous line refers to negative expression while interrupted line refers to positive expression. (A) Plasma membrane expression of MCT1; (B) Plasma membrane expression of MCT4.
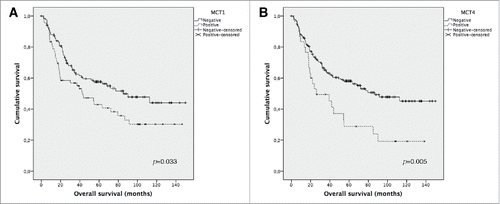
Table 5. Prognostic factors for overall survival in melanomas. Only expression in primary tumors was considered for MCT1, MCT4, GLUT1 and CAIX variables.
Discussion
Although recent advances have improved the management of patients with melanoma, with, for example, the use of specific inhibitors targeting mutated BRAF, resistance to the agents recently approved is a common event.Citation8,32 Importantly, V600E BRAF mutation was shown to drive the Warburg effect,Citation19 suggesting a relevance of the glycolytic metabolism in melanoma and a possible relation with response/resistance to BRAF inhibitors. Therefore, the knowledge on the expression and clinicopathological significance of key metabolism-related proteins in melanomas may bring new insights for melanoma patients' handling.
In the present study, the immunohistochemical evaluation showed that the expression of the metabolism-related proteins in primary melanomas varied from around 10% for GLUT1 to around 35% for MCT1, while, in metastases, expression frequencies varied from around 20% for GLUT1 to around 45% for MCT1, with similar results among lymph node and distant metastases (exception for CAIX expression). To the best of our knowledge, only one study evaluated the expression of MCT1 and MCT4 in melanomas; however, median score values were used instead of expression frequencies, making comparisons difficult to establish.Citation28 Regarding GLUT1 expression, more studies are available and, although 2 previous studies, with small casuistic and no description of melanoma histological type or anatomical site, showed lack of GLUT1 expression in primary melanomas,Citation33,34 a more recent study by Koch and collaborators showed an important GLUT1 expression in melanomas, with an expression frequency of 50% in primary tumors, 5 times the one found in the present study, and an expression frequency of 58% in metastases,Citation35 3 times the one found in the present study. These higher expression frequencies may be due to the different immunohistochemical classification, as only staining intensity was evaluated (with weak results also considered positive) and distinct cellular locations were not discriminated.Citation35 In the case of CAIX, a previous study showed lack of expression in melanomas, which is not in agreement with the findings of the present study.Citation36 However, once again, the size of the casuistic was small (only 32 malignant melanomas analyzed), with no description of melanoma histological types or anatomical site, while the use of different protocols during immunohistochemical procedure and different parameters to score the positive reactions may also contribute to these discordant results.
Importantly, MCT4 and GLUT1 expression was significantly increased in the transition from primary tumor to lymph-node metastases, suggesting that the hyperglycolytic phenotype, determined by increased glucose uptake by GLUT1 and lactate efflux by MCT4, contributes to the invasive capacity of cancer cells, as previously suggested by others.Citation1 These results are partially in agreement with the work of Ho and collaborators, where a significant increase in MCT1 expression levels when comparing metastases with thin primary melanomas was observed, as well as a significant increase in MCT4 expression levels when comparing primary melanomas or metastases with nevi, with no differences between primary melanomas and metastases.Citation28 In the present study, we did not observe an increase in MCT1 expression frequency in metastatic samples; however, Ho and collaborators observed a significant difference in primary versus metastatic melanomas when considering only thin primary melanomas. This division among primary tumors was not performed in the present study and may explain similar results obtained for MCT1 among primary and metastatic melanomas. The different results obtained regarding MCT4, may be due to sample size, as Ho and collaborators analyzed MCT4 expression in 31 primary melanomas, subdivided in thin and thick melanomas, decreasing the power of the statistical analysis due to small sample size. Additionally, Koch and collaborators showed a significant increase in GLUT1 expression in metastatic samples when compared with primary melanoma,Citation35 which is in accordance with the present study. As mentioned above, both MCT4 and GLUT1 are involved in the Warburg effect; while both these proteins allow the continuous flow of the glycolytic pathway by providing, respectively, efflux of intracellular lactate and glucose uptake by cancer cells, MCT4 also acts as a pH regulator by promoting proton efflux. As a result, the increased expression of these proteins in metastatic samples suggests a metabolic remodeling toward a hyperglycolytic and acid-resistant phenotype in the progression to an invasive phenotype, which is in accordance with evidence obtained from different approaches.Citation29
In the case of CAIX, a significant increase was observed in 2 transitions along melanoma progression: from benign nevi to primary tumor and from lymph-node metastasis to distant metastases. CAIX is a HIF-1α-induced pH regulator that contributes to the acid-mediated cancer cell invasive phenotype,Citation37-40 and has been associated with poor prognosis in a variety of neoplasias.Citation40 As mentioned above, only one additional study evaluated the expression of CAIX in melanoma samples, showing negative expression for this protein.Citation36 In the present study, the finding of an increased expression of CAIX along progression to malignancy is in accordance with the role of CAIX in mediating cancer cell invasive phenotype. Therefore, additional studies evaluating the expression of CAIX in malignant melanomas are warranted to further understand the actual contribution of this protein for melanoma progression.
Interestingly, both MCT1 and GLUT1 expression frequency was associated with BRAF mutation, in accordance with the observation that mutated BRAF drives the Warburg effect.Citation19 In the case of GLUT1, this association may be a result of HIF-1α stabilization by mutated BRAF,Citation20,21 as GLUT1 is induced by HIF-1α activity as a transcription factor,Citation41 while the same mechanism is not able to explain MCT1 association, as this MCT isoform is not a direct target of HIF-1α.Citation42 In fact, since MCT4 and CAIX are also targets of HIF-1α,Citation38,42 one would expect that these proteins would also be associated with BRAF mutation; however, regulation of these proteins is complex, with some mechanisms still to be described.
The metabolic reprograming of cancer cells, besides contributing to cancer cell growth under intermittent hypoxia, is involved in cancer cell aggressiveness and therapeutic resistance, and, consequently, patients' poor prognosis.Citation43 In this context, lactate emerges as an important player, through modulation of the tumor microenvironment.Citation5 As a result, MCTs may be associated with cancer patients' poor prognosis.Citation6 In melanoma, no previous study evaluated the clinicopathological significance of MCTs; however, an in vitro study showed that inhibition of MCTs, in particular MCT1, may be an effective therapeutic approach for malignant melanomas.Citation44 In the present study, both MCT isoforms, but to a higher extent MCT4, were significantly associated with different variables of poor prognosis, including overall survival. This is in accordance with the biological role of MCTs and tumor microenvironment modulation by lactate. However, it is important to mention that, although MCTs were significantly associated with overall survival in Kaplan-Meier and univariate Cox regression analysis, multivariate analysis showed that none of these proteins have a stage-independent prognostic value. Nevertheless, these results suggest that MCTs are promising druggable targets for melanoma treatment. Importantly, a Phase I clinical trial using a MCT1 specific inhibitor in patients with advanced prostate cancer, gastric cancer or diffuse large B cell lymphoma is currently recruiting participants (CRUKD/12/004). GLUT1 detains an important role in cancer metabolic reprogramming, as the high glycolytic flow requires high glucose uptake rates, mostly provided by this glucose transporter in cancer cells.Citation45 In accordance, in vitro suppression of GLUT1 in melanomas cells reduced proliferation, apoptosis repression, and migration, while in vivo suppression of GLUT1 reduced metastases formation.Citation35 Information regarding the prognostic value of GLUT1 using melanoma tissues is lacking; however, in the present study, we observed that GLUT1, at a lesser extent than MCTs, was associated with poor prognostic variables, in agreement with the role of this protein in the metabolic shift. Finally, CAIX does not appear to have a significant role in the prognosis of melanomas.
In conclusion, the present study brings new insights on the metabolic alterations of melanoma in the progression to a metastatic phenotype, showing an increased expression of MCT4 and GLUT1 in melanoma metastases. Also, we identified a clinicopathological value of MCT1, MCT4 and GLUT1 in melanoma. Since studies in this field are limited for melanomas, these results contribute to the characterization of melanoma molecular heterogeneity, paving the way for new options in the development of targeted therapies.
Patients and methods
Melanoma patient characteristics
The present series included 356 melanoma patients, treated from 1999 to 2012 at the Barretos Cancer Hospital, Barretos, SP, Brazil. Clinicopathological features included age at diagnosis (mean (SD): 58.3 (16.3) years), gender, clinical stage, Clark's level, Breslow's thickness (mean (SD): 4.1 (4.4) mm), histological subtype, anatomical site, number of mitoses per mm2, ulceration, peritumoral lymphocyte infiltration, intratumoral lymphocyte infiltration, tumor regression, pT, pN, pM, locoregional recurrence and overall survival. The main demographics and clinical data are depicted in . Patients were mainly treated with surgery (stages 0-III). Non-operable stage III/IV or recurrent disease were treated with cytotoxic chemotherapy (first line dacarbazine and second line carboplatin plus paclitaxel regiments). Bulky stage III operated tumors received adjuvant radiation at the physician description. Palliative radiation was indicated for central nervous system metastasis and symptomatic bone metastasis. No patient received adjuvant or target/immune therapy.
Table 6. Main demographics, clinical and histological characteristics of melanoma patients.
Human samples
Formalin-fixed paraffin-embedded (FFPE) melanoma samples (282 primary tumors, 117 lymph node metastases and 54 distant metastases; sample type distribution among patients is shown in Supplementary ) as well as 20 benign nevi samples were retrieved from the files of the Pathology Department of Barretos Cancer Hospital. The Barretos Cancer Hospital's Ethics Committee approved the present study (548/2011).
BRAF and NRAS mutations
DNA was obtained from FFPE tissue sections, as previously described.Citation46 Briefly, serially 10 μm thick unstained sections of paraffin blocks were sectioned and one H&E section was first evaluated by a pathologist to confirm the diagnosis and used for identification and selection of the areas of interest, which were macrodissected into a microfuge tube using a sterile needle (BD PrecisionGlide, BD, #305165). The macrodissected tissue was deparaffinized by a serial wash with xylol and ethanol (100%-70%-50%). DNA was extracted using Qiagen's QIAamp® DNA Micro Kit (Qiagen, #56304) following manufacturer's instructions and quantified by NanoDrop→ 2000 (Thermo Scientific).
The analysis of BRAF V600E mutation was performed by PCR followed by direct Sanger sequencing, as previously described.Citation47,48 PCR primers were as follows: 5′-AGTGGATTCGCGGGCACAGA-3′ (forward) and 5′-CAGCGCTGCCTGAAACTC-3′ (reverse). PCR cycling conditions were as follows: initial denaturation at 96°C for 15 minutes, followed by 40 cycles of 96°C denaturation for 45 seconds, annealing temperature at 55.5°C for 45 seconds and 72°C elongation for 45 seconds, and 72°C final elongation for 10 minutes, in a Verity PCR machine (Applied Biosystems).
The analysis of hotspot mutations of NRAS (codon 12/13 and 61) was performed by PCR followed by direct Sanger sequencing, as previously described.Citation47,48 The specific primers designed to include the regions of interest were as follows: 5′-ATGACTGAGTACAAACTGGT-3′ (forward) and 5′-CTCTATGGTGGGATCATATT-3′ (reverse) for codon 12/13, and 5′-TCTTACAGAAAACAAGTGGT-3′ (forward) and 5′-GTAGAGGTTAATATCCGCAA-3′ (reverse) for codon 61. The PCR cycling conditions were as follows: initial denaturation at 95°C for 15 minutes, followed by 40 cycles of 95°C denaturation for 45 seconds, annealing at 56.5°C for 45 seconds and 72°C elongation for 45 seconds, and 72°C final elongation for 10 minutes, in a Verity PCR machine (Applied Biosystems).
Amplification of PCR products was confirmed by gel electrophoresis. PCR products were purified using ExoSAP-IT (USB Corporation, #78200) and sequencing PCR was performed using a Big Dye terminator v3.1 cycle sequencing ready reaction kit (Applied Biosystems, #4337456) and the ABI PRISM 3500 xL Genetic Analyzer (Applied Biosystems). All mutated cases were confirmed with a second independent PCR followed by sequencing.
Immunohistochemistry
For immunohistochemical analysis, samples were organized into tissue microarrays (TMA) containing cores of 1.0 mm diameter. Each case was represented in TMAs by 3 cores and control samples (placenta and liver) were also included for TMA orientation. MCT1 immunohistochemistry was performed according to the avidin-biotin-peroxidase complex method (R.T.U. VECTASTAIN Elite ABC Kit (Universal), Vector Laboratories, #PK-7200), as previously described.Citation49 Immunohistochemistry for MCT4, GLUT1, and CAIX was performed according to the streptavidin-biotin-peroxidase complex principle (Ultravision Detection System Anti-polyvalent, HRP, Thermo Scientific, #TP-125-HL), as previously described.Citation50-52 Specificity of the antibodies was previously validated by siRNA followed by Western-blot.Citation53,54 Negative controls were performed by the use of appropriate serum controls for the primary antibodies (Dako, #N1698 and #N1699). Colon carcinoma tissue was used as positive control for MCT1 and MCT4, head and neck squamous cell carcinoma was used for GLUT1, and normal stomach was used for CAIX. Tissue sections were counterstained with hematoxylin and permanently mounted. Please refer to Supplementary for detailed information on each antibody used.
Immunohistochemical evaluation
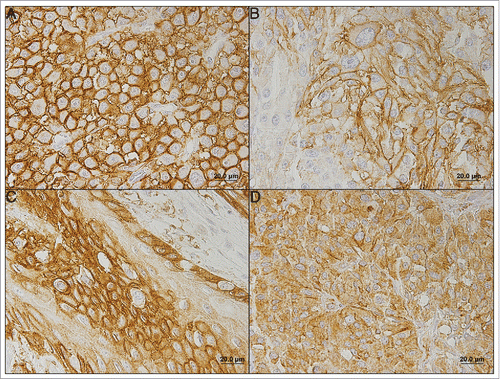
Protein expression was scored semi-quantitatively for plasma membrane expression in cancer cells as follows: 0: no immunoreactive cells; 1: < 5% of immunoreactive cells; 2: 5–50% of immunoreactive cells; and 3: > 50% of immunoreactive cells. Also, intensity of staining was scored semi-qualitatively as follows: 0: negative; 1: weak; 2: intermediate; and 3: strong. shows photomicrographs representative of staining intensity scores 1–3. The final score was defined as the sum of both parameters (extension and intensity), and grouped as negative (score 0 and 2) and positive (score 3–6), as previously described.Citation49 Two independent observers performed the immunohistochemical evaluation blindly and discordant results were discussed in a double-head microscope to determine the final score.
Statistical analysis
Data were stored and analyzed using the IBM SPSS Statistics software (version 21, IBM Company). All comparisons were examined for statistical significance using Pearson's chi-square (χ2) test and Fisher's exact test (when n < 5). Overall survival was defined as the time from the date of primary diagnosis to death related to melanoma or last follow-up and overall survival curves were estimated by the method of Kaplan-Meier and data compared using the log-rank test. Stage 0 (melanoma in situ) cases were excluded from survival analysis. Predictive factors of prognosis were identified using Cox proportional hazards regression models, which were used to estimate hazard ratios (HR) and 95% confidence intervals in univariate and multivariate analysis. For multivariate analysis, variables that reached a p value < 0.1 at univariate analysis were included. The threshold for significant p values was established as p < 0.05.
Abbreviations
| CAIX | = | Carbonic anhydrase IX |
| GLUT1 | = | Glucose transporter 1 |
| HIF-1α | = | Hypoxia inducible factor 1 alpha |
| MCTs | = | Monocarboxylate transporter |
| TMA | = | Tissue microarray |
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Supplementary Files
Download Zip (75.9 KB)Funding
This work was supported by FAPESP grant to VLV (2012/04194-1) and CP (2015/25351-6). VMG received a doctoral fellowship (SFRH/BD/51997/2012) from Fundação para a Ciência e a Tecnologia (FCT) and ON.2 SR&TD Integrated Program (NORTE-07-0124-FEDER-000017) co-funded by Programa Operacional Regional do Norte (ON.2- O Novo Norte), Quadro de Referência Estratégico Nacional (QREN), through Fundo Europeu de Desenvolvimento Regional (FEDER).
References
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004; 4:891-9; PMID:15516961; http://dx.doi.org/10.1038/nrc1478
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Granja S, Pinheiro C, Reis RM, Martinho O, Baltazar F. Glucose addiction in cancer therapy: advances and drawbacks. Curr Drug Metab 2015; In press; PMID:26504932
- Chiche J, Brahimi-Horn MC, Pouyssegur J. Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med 2010; 14:771-94; PMID:20015196; http://dx.doi.org/10.1111/j.1582-4934.2009.00994.x
- Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res 2011; 71:6921-5; PMID:22084445; http://dx.doi.org/10.1158/0008-5472.CAN-11-1457
- Baltazar F, Pinheiro C, Morais-Santos F, Azevedo-Silva J, Queiros O, Preto A, Casal M. Monocarboxylate transporters as targets and mediators in cancer therapy response. Histol Histopathol 2014; 29:1511-24; PMID:24921258;
- Erdmann F, Lortet-Tieulent J, Schuz J, Zeeb H, Greinert R, Breitbart EW, Bray F. International trends in the incidence of malignant melanoma 1953-2008–are recent generations at higher or lower risk? Int J Cancer 2013; 132:385-400; PMID:22532371; http://dx.doi.org/10.1002/ijc.27616
- Niezgoda A, Niezgoda P, Czajkowski R. Novel Approaches to Treatment of Advanced Melanoma: A Review on Targeted Therapy and Immunotherapy. Biomed Res Int 2015; 2015:851387; PMID:26171394; http://dx.doi.org/10.1155/2015/851387
- Safaee Ardekani G, Jafarnejad SM, Tan L, Saeedi A, Li G. The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis. PLoS One 2012; 7:e47054; PMID:23056577; http://dx.doi.org/10.1371/journal.pone.0047054
- Holderfield M, Deuker MM, McCormick F, McMahon M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer 2014; 14:455-67; PMID:24957944; http://dx.doi.org/10.1038/nrc3760
- Ugurel S, Thirumaran RK, Bloethner S, Gast A, Sucker A, Mueller-Berghaus J, Rittgen W, Hemminki K, Becker JC, Kumar R, et al. B-RAF and N-RAS mutations are preserved during short time in vitro propagation and differentially impact prognosis. PLoS One 2007; 2:e236; PMID:17311103; http://dx.doi.org/10.1371/journal.pone.0000236
- Bloom KJ, Vallera DU, Rueschoff J, Schilling R, Barbara Kovach Ba AS, Ochoa P, Langland R, Halait H, Dugan MC. Multisite analytic performance studies of a real-time polymerase chain reaction assay for the detection of BRAF V600E mutations in formalin-fixed, paraffin-embedded tissue specimens of malignant melanoma. Arch Pathol Lab Med 2012; 136:1385; PMID:22332713; http://dx.doi.org/10.5858/arpa.2011-0505-OA
- Goydos JS, Mann B, Kim HJ, Gabriel EM, Alsina J, Germino FJ, Shih W, Gorski DH. Detection of B-RAF and N-RAS mutations in human melanoma. J Am Coll Surg 2005; 200:362-70; PMID:15737846; http://dx.doi.org/10.1016/j.jamcollsurg.2004.10.032
- van Elsas A, Zerp SF, van der Flier S, Kruse KM, Aarnoudse C, Hayward NK, Ruiter DJ, Schrier PI. Relevance of ultraviolet-induced N-ras oncogene point mutations in development of primary human cutaneous melanoma. Am J Pathol 1996; 149:883-93; PMID:8780392
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507-16; PMID:21639808; http://dx.doi.org/10.1056/NEJMoa1103782
- Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012; 366:707-14; PMID:22356324; http://dx.doi.org/10.1056/NEJMoa1112302
- Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012; 367:107-14; PMID:22663011; http://dx.doi.org/10.1056/NEJMoa1203421
- Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012; 367:1694-703; PMID:23020132; http://dx.doi.org/10.1056/NEJMoa1210093
- Hall A, Meyle KD, Lange MK, Klima M, Sanderhoff M, Dahl C, Abildgaard C, Thorup K, Moghimi SM, Jensen PB, et al. Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the (V600E)BRAF oncogene. Oncotarget 2013; 4:584-99; PMID:23603840; http://dx.doi.org/10.18632/oncotarget.965
- Zerilli M, Zito G, Martorana A, Pitrone M, Cabibi D, Cappello F, Giordano C, Rodolico V. BRAF(V600E) mutation influences hypoxia-inducible factor-1alpha expression levels in papillary thyroid cancer. Mod Pathol 2010; 23:1052-60; PMID:20473281; http://dx.doi.org/10.1038/modpathol.2010.86
- Kumar SM, Yu H, Edwards R, Chen L, Kazianis S, Brafford P, Acs G, Herlyn M, Xu X. Mutant V600E BRAF increases hypoxia inducible factor-1alpha expression in melanoma. Cancer Res 2007; 67:3177-84; PMID:17409425; http://dx.doi.org/10.1158/0008-5472.CAN-06-3312
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell 2008; 13:472-82; PMID:18538731; http://dx.doi.org/10.1016/j.ccr.2008.05.005
- Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, Schmidt K, Willson JK, Markowitz S, Zhou S, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 2009; 325:1555-9; PMID:19661383; http://dx.doi.org/10.1126/science.1174229
- Gaglio D, Metallo CM, Gameiro PA, Hiller K, Danna LS, Balestrieri C, Alberghina L, Stephanopoulos G, Chiaradonna F. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol 2011; 7:523; PMID:21847114; http://dx.doi.org/10.1038/msb.2011.56
- Chiaradonna F, Sacco E, Manzoni R, Giorgio M, Vanoni M, Alberghina L. Ras-dependent carbon metabolism and transformation in mouse fibroblasts. Oncogene 2006; 25:5391-404; PMID:16607279; http://dx.doi.org/10.1038/sj.onc.1209528
- Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012; 149:656-70; PMID:22541435; http://dx.doi.org/10.1016/j.cell.2012.01.058
- Scott DA, Richardson AD, Filipp FV, Knutzen CA, Chiang GG, Ronai ZA, Osterman AL, Smith JW. Comparative metabolic flux profiling of melanoma cell lines: beyond the Warburg effect. J Biol Chem 2011; 286:42626-34; PMID:21998308; http://dx.doi.org/10.1074/jbc.M111.282046
- Ho J, de Moura MB, Lin Y, Vincent G, Thorne S, Duncan LM, Hui-Min L, Kirkwood JM, Becker D, Van Houten B, et al. Importance of glycolysis and oxidative phosphorylation in advanced melanoma. Mol Cancer 2012; 11:76; PMID:23043612; http://dx.doi.org/10.1186/1476-4598-11-76
- Bettum IJ, Gorad SS, Barkovskaya A, Pettersen S, Moestue SA, Vasiliauskaite K, Tenstad E, Oyjord T, Risa O, Nygaard V, et al. Metabolic reprogramming supports the invasive phenotype in malignant melanoma. Cancer Lett 2015; 366:71-83; PMID:26095603; http://dx.doi.org/10.1016/j.canlet.2015.06.006
- Hersey P, Watts RN, Zhang XD, Hackett J. Metabolic approaches to treatment of melanoma. Clin Cancer Res 2009; 15:6490-4; PMID:19861452; http://dx.doi.org/10.1158/1078-0432.CCR-09-0251
- Smith LK, Rao AD, McArthur GA. Targeting metabolic reprogramming as a potential therapeutic strategy in melanoma. Pharmacol Res 2016; 107:42-7; PMID:26924126; http://dx.doi.org/10.1016/j.phrs.2016.02.009
- Zhang W. BRAF inhibitors: the current and the future. Curr Opin Pharmacol 2015; 23:68-73; PMID:26072431; http://dx.doi.org/10.1016/j.coph.2015.05.015
- Carvalho KC, Cunha IW, Rocha RM, Ayala FR, Cajaiba MM, Begnami MD, Vilela RS, Paiva GR, Andrade RG, Soares FA. GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics (Sao Paulo) 2011; 66:965-72; PMID:21808860; http://dx.doi.org/10.1590/S1807-59322011000600008
- Baer SC, Casaubon L, Younes M. Expression of the human erythrocyte glucose transporter Glut1 in cutaneous neoplasia. J Am Acad Dermatol 1997; 37:575-7; PMID:9344196; http://dx.doi.org/10.1016/S0190-9622(97)70174-9
- Koch A, Arke Lang S, Johannes Wild P, Gantner S, Mahli A, Spanier G, Berneburg M, Muller M, Katrin Bosserhoff A, Hellerbrand C. Glucose transporter isoform 1 expression enhances metastasis of malignant melanoma cells. Oncotarget 2015; 6:32748-60; PMID:26293674
- Syrjanen L, Luukkaala T, Leppilampi M, Kallioinen M, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila S, Karttunen T. Expression of cancer-related carbonic anhydrases IX and XII in normal skin and skin neoplasms. APMIS 2014; 122:880-9; PMID:24698175; http://dx.doi.org/10.1111/apm.12251
- Smallbone K, Gavaghan DJ, Gatenby RA, Maini PK. The role of acidity in solid tumour growth and invasion. J Theor Biol 2005; 235:476-84; PMID:15935166; http://dx.doi.org/10.1016/j.jtbi.2005.02.001
- Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 2000; 60:7075-83; PMID:11156414
- Shin HJ, Rho SB, Jung DC, Han IO, Oh ES, Kim JY. Carbonic anhydrase IX (CA9) modulates tumor-associated cell migration and invasion. J Cell Sci 2011; 124:1077-87; PMID:21363891; http://dx.doi.org/10.1242/jcs.072207
- Chiche J, Ilc K, Brahimi-Horn MC, Pouyssegur J. Membrane-bound carbonic anhydrases are key pH regulators controlling tumor growth and cell migration. Adv Enzyme Regul 2010; 50:20-33; PMID:19895836; http://dx.doi.org/10.1016/j.advenzreg.2009.10.005
- Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol 2005; 202:654-62; PMID:15389572; http://dx.doi.org/10.1002/jcp.20166
- Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 2006; 281:9030-7; PMID:16452478; http://dx.doi.org/10.1074/jbc.M511397200
- Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer 2010; 10:267-77; PMID:20300106; http://dx.doi.org/10.1038/nrc2817
- Wahl ML, Owen JA, Burd R, Herlands RA, Nogami SS, Rodeck U, Berd D, Leeper DB, Owen CS. Regulation of intracellular pH in human melanoma: potential therapeutic implications. Mol Cancer Ther 2002; 1:617-28; PMID:12479222
- Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther 2009; 121:29-40; PMID:18992769; http://dx.doi.org/10.1016/j.pharmthera.2008.09.005
- Yamane LS, Scapulatempo-Neto C, Alvarenga L, Oliveira CZ, Berardinelli GN, Almodova E, Cunha TR, Fava G, Colaiacovo W, Melani A, et al. KRAS and BRAF mutations and MSI status in precursor lesions of colorectal cancer detected by colonoscopy. Oncol Rep 2014; 32:1419-26; PMID:25050586
- de Lima Vazquez V, Vicente AL, Carloni A, Berardinelli G, Soares P, Scapulatempo C, Martinho O, Reis RM. Molecular profiling, including TERT promoter mutations, of acral lentiginous melanomas. Melanoma Res 2016; 26:93-9; PMID:26709572; http://dx.doi.org/10.1097/CMR.0000000000000222
- Martinho O, Gouveia A, Viana-Pereira M, Silva P, Pimenta A, Reis RM, Lopes JM. Low frequency of MAP kinase pathway alterations in KIT and PDGFRA wild-type GISTs. Histopathology 2009; 55:53-62; PMID:19614767; http://dx.doi.org/10.1111/j.1365-2559.2009.03323.x
- Pinheiro C, Longatto-Filho A, Scapulatempo C, Ferreira L, Martins S, Pellerin L, Rodrigues M, Alves VA, Schmitt F, Baltazar F. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch 2008; 452:139-46; PMID:18188595; http://dx.doi.org/10.1007/s00428-007-0558-5
- Pinheiro C, Sousa B, Albergaria A, Paredes J, Dufloth R, Vieira D, Schmitt F, Baltazar F. GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol 2011; 26:1279-86; PMID:21870331
- Queiros O, Preto A, Pacheco A, Pinheiro C, Azevedo-Silva J, Moreira R, Pedro M, Ko YH, Pedersen PL, Baltazar F, et al. Butyrate activates the monocarboxylate transporter MCT4 expression in breast cancer cells and enhances the antitumor activity of 3-bromopyruvate. J Bioenerg Biomembr 2012; 44:141-53; PMID:22350013; http://dx.doi.org/10.1007/s10863-012-9418-3
- Pinheiro C, Reis RM, Ricardo S, Longatto-Filho A, Schmitt F, Baltazar F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J Biomed Biotechnol 2010; 2010:427694; PMID:20454640; http://dx.doi.org/10.1155/2010/427694
- Morais-Santos F, Granja S, Miranda-Gonçalves V, Moreira AHJ, Queirós S, Vilaça Jl, Schmitt FC, Longatto-Filho A, Paredes J, Baltazar F, et al. Targeting lactate transport suppresses in vivo breast tumour growth. Oncotarget 2015; PMID:26203664
- Sousa B, Ribeiro AS, Nobre AR, Lopes N, Martins D, Pinheiro C, Vieira AF, Albergaria A, Gerhard R, Schmitt F, et al. The basal epithelial marker P-cadherin associates with breast cancer cell populations harboring a glycolytic and acid-resistant phenotype. BMC Cancer 2014; 14:734; PMID:25269858; http://dx.doi.org/10.1186/1471-2407-14-734