Glioblastoma multiforme (GBM) is the most common and lethal primary brain tumor in adults, which displays significant heterogeneity within the tumor mass. A subpopulation of cells (6%–29%) called glioma-initiating cells (GICs) are responsible for tumorigenesis and resistance to conventional therapies.Citation1 The hypoxia microenvironment serves as an essential niche for GICs that promotes their self-renewal and tumorigenic potential through induction of stem cell markers such as OCT4 by the hypoxia inducible factors (HIF).Citation2 Besides stemness, multiple aspects of GICs need to be modulated to reprogram cells to a state favorable for residing in the hypoxic environment and maintaining their distinct biological features, which are yet largely unknown. We recently reported a miRNA-KDM1B mediated signaling that is critical to reprogram GICs for adaptation to hypoxia.Citation3
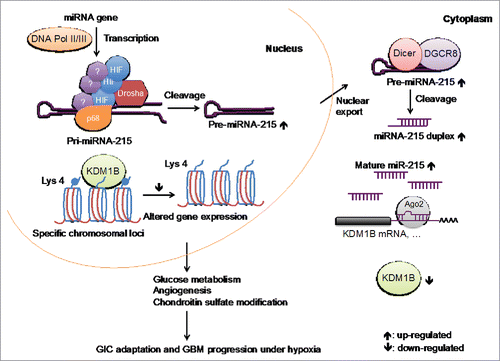
Through a microRNA qPCR array in GICs isolated from patient-derived GBM xenografts and a functional screen in mice, the hypoxia-induced miR-215 was identified as a pivotal regulator for the response of GICs to the hypoxic stress, since antagonizing function of miR-215 impeded GICs growth under hypoxia and intracranial tumor formation in the immune-compromised mice. As a master transcriptional factor during hypoxia, HIFs transcriptionally modulate multiple processes associated with GBM progression. Surprisingly, expression profiling of the mature miRNAs as well as the pri- and pre-miRNAs, which are the intermediates of the miRNA biogenesis pathway, revealed that the processing of miR-215 and several other hypoxia-induced miRNAs was boosted post-transcriptionally from the cleavage step mediated by the Drosha/DGCR8 complex. Enhanced interaction between HIFs and Drosha was observed in GICs cultured under hypoxia, accelerating the recruitment of HIF-Drosha complex on specific pri-miRNAs to promote their processing activities. Besides HIFs, 2 other transcription factors, Smads and p53, have been demonstrated to enhance post-transcriptional miRNA biogenesis through a similar manner.Citation4 These findings indicate that transcription factors may in general play multi-faceted functions. It is still unknown how HIFs interact with the specific pri-miRNAs. Unlike smad, HIF could not bind pri-miRNAs directly through its DNA binding domain. It is possible that HIFs may bind pri-miRNAs directly with other domains or indirectly through association with unknown mediators. Notably, the induction of miR-215 by hypoxia presented only in GICs rather than paired non-GICs, suggesting specific regulations of miR-215 biogenesis by hypoxia in GICs. These questions need to be further addressed.
Mechanistically, miR-215 acts through suppressing the expression of KDM1B, since GIC growth defects mediated by miR-215 blockage under hypoxia could be rescued by knocking down of KDM1B. KDM1B is a FAD-dependent histone demethylase that demethylates mono- and di-methylated histone H3K4, thereby possessing a great potential to modulate the expression of a large number of genes. Through RNA-Seq analysis in GICs with knocking down of KDM1B, we observed that genes associated with multiple pathways were regulated by the hypoxia-miR-215-KDM1B axis, including glucose metabolism, angiogenesis and biosynthesis of chondroitin sulfate proteoglycan (CSPG). We further examined the activities of those pathways and demonstrated that miR-215-KDM1B activated the anaerobic switch that favored glycolysis and promoted the angiogenic activity of GICs during hypoxic responses. Moreover, the enzymes catalyzing the biosynthesis pathway of CPSG were identified as novel regulators altered by hypoxia via miR-215-KDM1B. Blocking the synthesis of CSPG chains by chondroitinase ABC dramatically dampened the GICs abilities to grow and self-renew. CSPGs have been reported to promote tumorigenesis through interaction with chemokines and growth factors in the ECM.Citation5,6 In addition, CPSG acts as a central organizer of the tumor microenvironment that induces reactive astrocytes withdraw from the edge of the tumor mass and activates tumor-associated microglia.Citation7 It is possible that accumulated CSPGs in the hypoxic region facilitate tumor growth though activating the growth factor mediated signal pathways and creating a microenvironment barrier that prevents the infiltration of unfavorable cells such as immune cells. The detailed mechanisms how CSPGs mediate glioma progression remain to be investigated. On the other hand, it is still puzzling how KDM1B regulates gene expression epigenetically. Total levels of H3K4me1/2 were not altered in GICs cultured under hypoxia. A global down-regulation or up-regulation of gene expression was not observed, while the expression of specific genes contributing to the functions of KDM1B in GICs, such as HIF2α, Glut3 and VEGF, were mainly elevated when KDM1B was knocked down. These notions indicate that the regulatory roles of KDM1B on the H3K4me1/2 motif might associate with specific chromosomal loci where other transcriptional or epigenetic factors may be involved.
Finally, we conducted analyses from GBM tissue microarray and published datasets and demonstrated that miR-215 expression was inversely correlated with KDM1B expression while positively associated with HIF1α expression and prognosis of GBM patients, strongly supporting the clinical significance of the hypoxia-miR-215-KDM1B signaling in GBM patients.
In summary, miR-215 is induced by hypoxia specifically in the GICs through HIF-Drosha interaction that enhances its post-transcriptional processing. The induction of miR-215 suppresses KDM1B, leading to alteration of multiple pathway activities and ultimately progression of GICs in the hypoxic niche (). These findings reveal a direct role of HIF in regulating microRNA biogenesis. In the meantime, identification of the HIF-miR-215-KDM1B axis required for GIC adaptation to hypoxia, and its significant clinical correlation in GBM patients may provide important basis for developing treatment against this signaling pathway.
Figure 1. HIF-miR-215-KDM1B signaling in mediating GICs' responses to hypoxia. During hypoxia in GICs, the biogenesis of miR-215 is accelerated post-transcriptionally by HIFs through enhancement in the formation and recruitment of HIF-Drosha complex on pri-miR-215. The induction of miR-215 acts to reprogram GICs for adaptation to hypoxia via suppressing the expression of KDM1B, which modulates the activities of multiple pathways.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Singh SK, et al. Nature 2004; 432(7015):396-401; PMID:15549107; http://dx.doi.org/10.1038/Nature03128
- Li Z, et al. Cancer cell 2009; 15(6):501-13. PMID:19477429; http://dx.doi.org/10.1016/j.ccr.2009.03.018
- Hu J, et al. Cancer Cell 2016; 29(1):49-60. PMID:26766590; http://dx.doi.org/10.1016/j.ccell.2015.12.005
- Ha M, et al. Nat Rev Mol Cell Biol 2014; 15(8):509-24. PMID:25027649; http://dx.doi.org/10.1038/nrm3838
- Takada W, et al. Anal Biochem 2013; 435(2):123-30; PMID:23333222; http://dx.doi.org/10.1016/J.Ab.2013.01.004
- Hirose J, et al. J Biol Chem 2001; 276(7):5228-34; PMID:11083865; http://dx.doi.org/10.1074/jbc.M007542200
- Silver DJ, et al. J Neurosci 2013; 33(39):15603-17. PMID:24068827; http://dx.doi.org/10.1523/JNEUROSCI.3004-12.2013
