Hippo (Hpo) signaling is a conserved mechanism that controls organ growth. In Drosophila, activation of core protein kinases, Hpo and Warts (Wts), suppresses organ growth by phosphorylating the Yorkie (Yki) transcriptional coactivator (YAP in mammals). Conversely, unphosphorylated Yki can enter the nucleus to induce the expression of target genes cyclin E and Diap1 to facilitate cell cycle progression and inhibit apoptosis, respectively. One of the central issues in Hippo signaling is to understand how the Hpo-Wts kinase cascade is activated. Several factors have been identified that function upstream of Hpo. Among these factors are Crumbs (Crb) and Fat (Ft) which are membrane proteins that regulate the localization of a FERM (4.1-Ezrin-Radixin-Moesin) domain protein Expanded (Ex) to the cell membrane.Citation1 Another protein kinase Tao-1 acts downstream of Ex to phosphorylate Hpo.Citation2-3 However, the details on how the function of Ex is linked to Tao-1 remained to be a puzzle.
Recently, we have identified Schip1, the Drosophila homolog of human Schwannomin-interacting protein-1 (SCHIP-1), as a new component of Hippo signaling. Loss of Schip1 results in overproliferation by up-regulating Yki target genes, indicating its role in the regulation of Hpo-Yki signaling. Importantly, Schip1 is directly involved in linking Ex and Tao-1 kinase.Citation4 Schip1 partially co-localizes with Ex to the apical cell membranes in an Ex-dependent manner. Further, Schip1 is required for the localization of cytoplasmic Tao-1 kinase to the cell membrane to promote the Hpo function. In addition, Schip1 directly interacts with Ex as well as Tao-1 and promotes Tao-1 kinase activity to phosphorylate Hpo, thus supporting its role as a functional linker between Ex and Tao-1 ().
Figure 1. A model for Schip1 function in Hippo signaling in Drosophila. Crb, Ex and Mer are associated with apical cell membrane. Ex directly recruits Schip1, and Schip1 interacts with Tao-1 to facilitate Hpo phosphorylation.Citation4 Mer acts upstream to Hpo but can also recruit Wts.Citation7 Schip1 binds to Mer, but it is unknown whether it affects Mer-Wts interaction. Schip1 may also be required for cell survival after larval growth (see text for details). AJ: Adherens Junction.
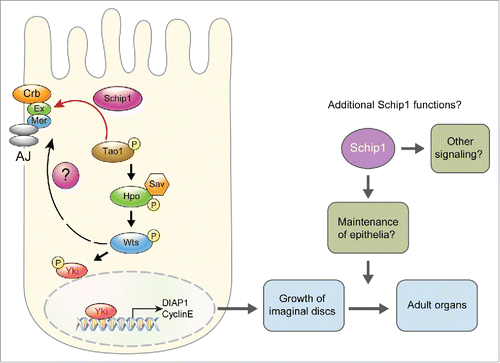
These findings of Schip1 function provide insights into the mechanism of Tao-1-dependent Hpo activation but also raise interesting questions. Firstly, it is yet unclear how Schip1 is related to another FERM domain protein Merlin (Mer), the Drosophila homolog of Schwannomin/NF2 associated with Ex. Human SCHIP-1 was initially identified from a two-hybrid screen as a binding partner of Schwannomin (NF2, human Merlin).Citation5 We have also confirmed that Schip1 in Drosophila interacts with the FERM domains of Mer as well as Ex. In addition, we found that reduction of Mer or Schip1 causes similar lateral expansion of the adult head. Furthermore, overexpression of Schip1 suppresses the head expansion caused by Mer knockdown.Citation5 Thus, Schip1 seems to function downstream of Mer or in parallel in head tissues. However, Ex and Mer are known to have partially redundant and specific functions in different cellular contexts.Citation1,6 Due to tissue specificity and potential redundancy of Mer function, additional work is necessary to clearly define the functional relationship between Schip1 and Mer in various organs. Detailed analysis of Mer-Schip1 interaction in Drosophila may provide valuable clues to the molecular basis of neurofibromatosis II tumors associated with human Merlin mutations.
Secondly, it is interesting to see whether Schip1 can regulate the activity of Wts in parallel with Tao-1, since Mer can directly interact with Wts.Citation7 It has been proposed that upon activation of Hpo signaling, Hpo and Wts are recruited to cell membranes where Wts is phosphorylated, leading to the inhibition of Yki activity. Now that Schip1 is a new factor that can directly interact with both Ex and Tao-1, Schip1 might play a dual role in mediating the localization of Wts as well as Hpo, either directly or indirectly through interacting with the FERM domains of Ex and Mer ().
Thirdly, despite overgrowth of Schip1 mutant tissues in imaginal discs, resulting organs in adults show relatively mild enlargement compared with the massive growth of hippo or wts mutant adult tissues.Citation4 Similar phenomena have also been seen in ex mutant organs. Several upstream regulators of Hippo signaling including Crb, Ex and Schip1 are localized to the apical membrane. As Crb is important for the integrity of epithelia, loss of Ex or Schip1 may impair disc epithelia, potentially affecting the organ size during later stages of development. In addition, Schip1 might also participate in signaling pathways other than Hippo signaling. For example, loss of Ex causes an increase in the Wingless (Wg) level in the eye disc. Because Wg up-regulation antagonizes retinal differentiation, ex mutation may cause combined effects of overproliferation and inhibition of retinal differentiation, reducing the eye size.Citation6 Thus, it is worth testing whether Schip1 is involved in mediating Wg regulation by Ex in eye disc or other signaling events.
In conclusion, Schip1 provides a critical molecular link that allows the activation of the core kinase cascade in the Hippo pathway. It remains to be determined whether this role of Schip1 is evolutionarily conserved in other organisms including humans. Loss of function analysis of mammalian SCHIP-1 homologs would be necessary to support their roles as tumor suppressors that negatively regulate the YAP oncogene.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development 2011; 138:9-22; PMID:21138973; http://dx.doi.org/10.1242/dev.045500
- Boggiano JC, Vanderzalm PJ, Gehon RG. Tao-1 phosphorylates hippo/MST kinases to regulate the hippo-Salvador-Warts tumor suppressor pathway. Dev Cell 2011; 21:888-95; PMID:22075147; http://dx.doi.org/10.1016/j.devcel.2011.08.028
- Poon CL, Lin JI, Zhang X, Harvey KF. The sterile 20-like kinase tao-1 controls tissue growth by regulating the Salvador-Warts-hippo pathway. The sterile 20-like kinase tao-1 controls tissue growth by regulating the Salvador-Warts-hippo pathway. Dev Cell 2011; 21:896-906; PMID:22075148; http://dx.doi.org/10.1016/j.devcel.2011.09.012
- Chung HL, Augustine GJ, Choi K-W. Drosophila Schip1 links expanded and tao-1 to regulate hippo signaling. Dev Cell 2016; 36:511-24; PMID:26954546; http://dx.doi.org/10.1016/j.devcel.2016.02.004
- Goutebroze L, Brault E, Muchardt C, Camonis J, Thomas G. Cloning and characterization of SCHIP-1, a novel protein interacting specifically with spliced isoforms and naturally occurring mutant NF2 proteins. Mol Cell Bio 2000; 20:1699-712; http://dx.doi.org/10.1128/MCB.20.5.1699-1712.2000
- Pellock BJ, Buff E, White K, Hariharan IK. The Drosophila tumor suppressors expanded and Merlin differentially regulate cell cycle exit, apoptosis, and wingless signaling. Dev Bio 2007; 304:102-15; http://dx.doi.org/10.1016/j.ydbio.2006.12.021
- Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 2013; 154:1342-55; PMID:24012335; http://dx.doi.org/10.1016/j.cell.2013.08.025
