ABSTRACT
Cell cycle checkpoint signaling stringently regulates chromosome segregation during cell division. MAD2 is one of the key components of the spindle and mitotic checkpoint complex that regulates the fidelity of cell division along with MAD1, CDC20, BUBR1, BUB3 and MAD3. MAD2 ablation leads to erroneous attachment of kinetochore-spindle fibers and defective chromosome separation. A potential role for MAD2 in the regulation of events beyond the spindle and mitotic checkpoints is not clear. Together with active spindle assembly checkpoint signaling, AURORA B kinase activity is essential for chromosome condensation as cells enter mitosis. AURORA B phosphorylates histone H3 at serine 10 and serine 28 to facilitate the formation of condensed metaphase chromosomes. In the absence of functional AURORA B cells escape mitosis despite the presence of misaligned chromosomes. In this study we report that silencing of MAD2 results in a drastic reduction of metaphase-specific histone H3 phosphorylation at serine 10 and serine 28. We demonstrate that this is due to mislocalization of AURORA B in the absence of MAD2. Conversely, overexpression of MAD2 concentrated the localization of AURORA B at the metaphase plate and caused hyper-phosphorylation of histone H3. We find that MAD1 plays a minor role in influencing the MAD2-dependent regulation of AURORA B suggesting that the effects of MAD2 on AURORA B are independent of the spindle checkpoint complex. Our findings reveal that, in addition to its role in checkpoint signaling, MAD2 ensures chromosome stability through the regulation of AURORA B.
Introduction
MAD2 is a critical member of the spindle assembly checkpoint (SAC) and mitotic checkpoint (MCC) complexes. At the onset of mitosis MAD2 and its interacting partner MAD1 bind to unattached kinetochores and activate SAC signaling to ensure proper spindle microtubule and kinetochore attachments and the fidelity of chromosome segregation.Citation1 MAD2 together with other checkpoint proteins CDC20, BUBR1, BUB3 and MAD3 form the MCC which blocks the function of Anaphase promoting complex/Cyclosome (APC/C). This pauses the cells in metaphase until all the chromosomes are properly attached and bi-oriented.Citation2,3
In addition to SAC- and MCC-based regulation of the cell cycle, several kinases and phosphatases also play important roles in guiding stringent chromosome segregation. AURORA B kinase is a member of the chromosome passenger complex (CPC) which associates with the inner centromeres and regulates chromosome separation during mitosis.Citation4 AURORA B targets histone H3 S10 and S28 and their phosphorylation marks the condensed metaphase chromosomes.Citation5 Recent studies have suggested a possible link between MAD2 and AURORA B function during mitosis.Citation6 However it is not clear how MAD2 directly regulates AURORA B function to promote proper chromosome separation and genomic stability. We show here that MAD2 is a critical mediator of chromosome segregation by regulating the mitotic localization and function of AURORA B. RNAi-mediated silencing of MAD2 results in aberrant localization of AURORA B at the metaphase plate and consequent loss of histone H3 phosphorylation. Our results also show that MAD2 (not MAD1) is the critical SAC component which modulates mitotic functions of AURORA B and is instrumental in preventing chromosome-segregation defects.
Results
MAD2 regulates AURORA B-mediated mitotic phosphorylation of histone H3
Our earlier work suggested that MAD2 might play a role in events leading to chromosome condensation during mitosis.Citation7 To study the effect of MAD2 on epigenetic changes linked to condensed metaphase chromosomesCitation8,9 we silenced MAD2 in HeLa cells and found that the phosphorylation status of histone H3 S10 and S28 residues was drastically reduced (). Immunofluorescence analysis of metaphase chromosomes in HeLa cells revealed mitotic specific reduction of histone H3 phosphorylation after MAD2 silencing (). RNAi-mediated silencing of AURORA B also resulted in a significant reduction of H3 S10 and S28 phosphorylation levels (). We next performed siRNA-rescue experiments to confirm the specificity of the observed effect on H3 phosphorylation. Our results showed that co-transfection of a MAD2 expression plasmidCitation10 (that is RNAi-resistant in our system) can rescue the phosphorylation of histone H3S28 in MAD2-silenced HeLa cells. The recovery in the level of histone H3 phosphorylation was comparable to the control cells ().
Figure 1. MAD2 regulates AURORA B-mediated mitotic phosphorylation of histone H3. (A). HeLa cells were transfected with control, MAD2 siRNA or AURORA B siRNA for 48 hours, followed by immunoblotting with anti-MAD2, anti-AURORA B and anti-TUBULIN antibodies for control. The phosphorylation status of histone H3 residues serine 10 and serine 28 were probed with phospho-site specific antibodies. Histone H3 Western blotting was performed as a control. (B). Immunofluorescence analysis of control and MAD2 siRNA transfected HeLa cells was performed with the specific antibodies to visualize phosphorylation status of histone H3 (S10 and S28) during metaphase. DNA was stained with Hoechst. (C). AURORA B siRNA transfected HeLa cells were analyzed as in B. Scale bar is 10 microns. (D). HeLa cells were transfected with either control or MAD2 siRNA for 48 hours. The control and siRNA transfected cells (24 hours after first transfection) were then co-transfected with either vector or MAD2 expression plasmid (that is RNAi-resistant) for another 24 hours. The cells were then immuno-blotted with anti-MAD2, anti-phospho-H3S28 and anti-TUBULIN antibodies. (E). HeLa cells and WiT49 cells were transfected with either control or MAD2 siRNA for 48 hours and Western blotting was done with anti-MAD2, anti-phospho-H3T3 and anti-TUBULIN antibodies.
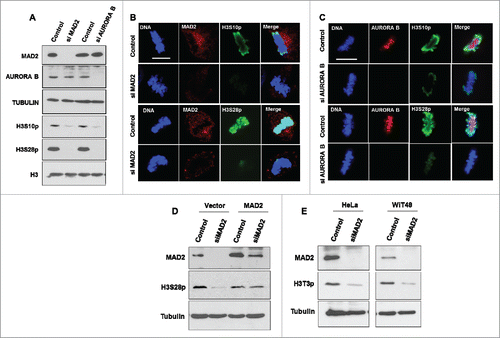
Similar to the observations above, our recent study showed that MAD2 knockdown in WiT49 and M15 cells also resulted in a drastic reduction in H3 S10 phosphorylation.Citation7 Interestingly, knockdown of either MAD2 or AURORA B in MCF7 cells had negligible effect on mitotic histone H3 S10 phosphorylation but still caused a significant reduction in phosphorylation of histone H3 S28 (Supplementary Fig. S1A), Taken together the results suggest cell type specific effects of MAD2 depletion on histone H3 phosphorylation.
At the onset of mitosis, histone H3 is known to be phosphorylated by HASPIN kinase at Threonine 3 residue. Phosphorylation of Histone H3T3 is crucial for the recruitment of AURORA B at the centromeres.Citation11,12 However, AURORA B has been shown to regulate the phosphorylation status of histone H3T3 via a feedback loop.Citation13,14 We therefore determined if MAD2 knockdown influences H3T3 phosphorylation. Interestingly, we found that MAD2 ablation resulted in a significant reduction of H3T3 phosphorylation in both HeLa and WiT49 cells (). This result shows that MAD2 most likely affects multiple signaling pathways by directly regulating AURORA B. Hence silencing MAD2 negatively affects AURORA B mediated phosphorylation of the histone H3 tail which is essential for mitotic chromosomal condensation.
MAD2 regulates AURORA B localization during mitosis
The expression and activity of AURORA B peaks during mitosis.Citation5 AURORA B along with 3 other proteins, SURVIVIN, BOREALIN and INCENP form the chromosome passenger complex (CPC).Citation4,15 Our results so far have shown that MAD2 ablation results in the reduced phosphorylation of histone H3. In order to identify the role of MAD2 in the regulation of mitotic localization of AURORA B, we silenced MAD2 in different cell lines and studied AURORA B localization using immunofluorescence analysis. Our results in HeLa, WiT49 () and MCF7 cells (Supplementary Fig. S1B) show that AURORA B occupancy at the metaphase plate is reduced upon MAD2 silencing.
Figure 2. MAD2 regulates AURORA B localization during mitosis. (A). HeLa and (B) WiT49 cells were transfected with control or MAD2 siRNA for 48 hours, followed by immunofluorescence analysis with anti-MAD2 and anti-AURORA B antibodies to visualize the mitotic localization of AURORA B during mitosis. DNA was stained with Hoechst. Scale bar is 10 microns. (C). Mitotic index showing percentage of HeLa cells in mitosis after treatment with either DMSO (control) or MG-132 (5 μM) for 4 hours (D). HeLa cells were transfected with control, MAD2 and AURORA B siRNA for 48 hours followed by treatment with either DMSO or MG-132 (5 µM) for 4 hours. Immunoblotting was performed in with anti-MAD2, anti-AURORA B and anti-TUBULIN antibodies. The phosphorylation status of histone H3 at serine 28 was probed using its phospho-site specific antibody. Histone H3 Western blotting was performed as a control. (E). HeLa cells and (F). WiT49 cells were transfected with control or MAD2 siRNA for 48 hours, followed by immunofluorescence analysis with anti-MAD2 and anti-SURVIVIN antibodies. DNA was stained with Hoechst. Scale bar is 10 microns.
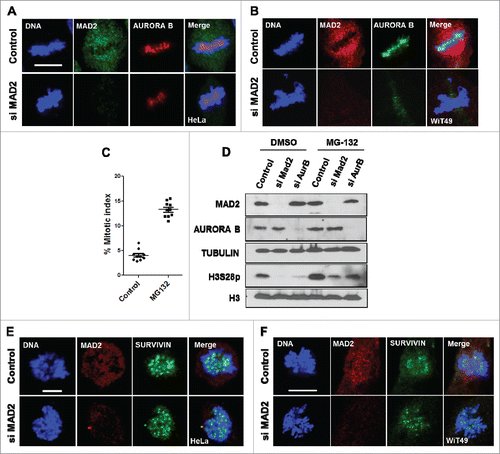
MAD2 ablation is known to promote early anaphase entry due to inactive mitotic checkpoint function, which might account for the effects that we have observed so far. To rule out this possibility we performed experiments in presence of the proteasome inhibitor MG-132 (5 μM) for 4 hoursCitation6 which effectively prevents mitotic exit and arrests the cells at metaphase (). The cells were transfected with either MAD2 siRNA or AURORA B siRNA (similar to that described in ), followed by a 4 hour treatment with either DMSO or MG-132 (5 μM). Our results show that delaying anaphase entry did not significantly change the effect of silencing MAD2 or AURORA B on histone H3 S28 phosphorylation (). Taken together, our data show that MAD2 has a profound effect on AURORA B recruitment and function during mitosis and provide strong evidence that AURORA B can be regulated by SAC signaling components. Interestingly, we found no significant defects in the mitotic localization of SURVIVIN (another member of CPC)Citation4,15 upon MAD2 silencing in HeLa and WiT49 cells (). This observation suggests that MAD2 may not influence the functional integrity of the CPC but rather affects specific properties of AURORA B.
MAD2 overexpression enhances AURORA B-mediated phosphorylation of histone H3 during mitosis
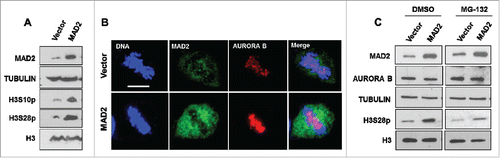
Silencing MAD2 leads to mislocalized AURORA B and reduced histone H3 phosphorylation during mitosis which promotes error-prone cell division. We next tested whether MAD2 overexpression conversely affects AURORA B function. shows that overexpression of MAD2 increased phosphorylation of histone H3 at both S10 and S28 residues. Moreover, HeLa cells transfected with MAD2 overexpression construct exhibited enhanced AURORA B signal at the metaphase plate when compared to the control transfected cells (). Again, blocking early mitotic exit using MG-132 (5 μM) for 4 hours had no significant effect on the elevation of histone H3 S28 hyper-phosphorylation in MAD2 overexpressing cells when compared to control DMSO-treated cells.
Figure 3. MAD2 overexpression enhances AURORA B-mediated phosphorylation of histone H3 during mitosis. (A). HeLa cells were transfected with an empty vector or MAD2 overexpression plasmid for 48 hours, followed by immunoblotting with anti-MAD2 and phosphorylation specific antibodies against histone H3 residues serine 10 and serine 28. Western blotting with histone H3 and TUBULIN antibodies were performed as controls. (B). HeLa cells were transfected with vector or MAD2 overexpression plasmid for 48 hours, followed by immunofluorescence analysis with anti-MAD2 and anti-AURORA B antibodies to visualize the mitotic localization of AURORA B during metaphase. DNA was stained with Hoechst. Scale bar is 10 microns. (C). HeLa cells were transfected with vector or MAD2 overexpression plasmid for 48 hours followed by treatment with either DMSO or MG-132 (5 µM) for 4 hours. Immunoblotting was performed in with anti-MAD2 and anti-AURORA antibodies. The phosphorylation status of histone H3 at serine 28 was probed using its phospho-site specific antibody. Western blotting with histone H3 and TUBULIN antibodies were performed as controls.
MAD1 and MAD2 complex regulates mitotic functions of AURORA B
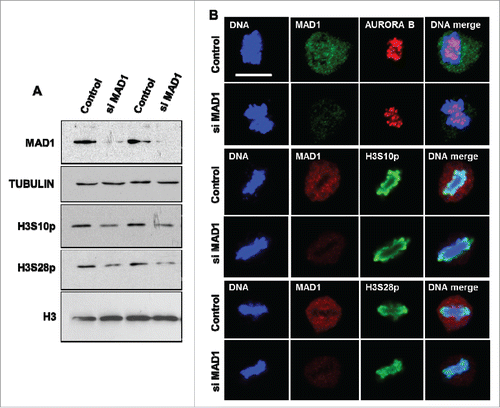
Our results so far have shown that perturbation in MAD2 protein level significantly alters the status of histone H3 phosphorylation and AURORA B occupancy during mitosis. MAD2 plays an important role in the initiation of spindle assembly checkpoint signaling along with MAD1 at the kinetochores. MAD1 recruits soluble O-MAD2 (open conformation of MAD2) and stimulates the conversion to C-MAD2 (closed active conformation of MAD2). During pro-metaphase the MAD1-MAD2 tetramer binds to the unattached kinetochores and amplifies SAC signaling.Citation1-3 In order to determine if MAD1-mediated initial recruitment of MAD2 is the critical step in regulating AURORA B function, we silenced MAD1 in HeLa cells and analyzed the phosphorylation levels of histone H3 (S10 and S28). Interestingly, loss of MAD1 resulted in only a minor reduction in the level of histone H3 phosphorylation (S10 or S28; ), when compared to the effect of MAD2 silencing (). Furthermore, immunofluorescence analysis of MAD1-silenced cells did not show a significant difference in either AURORA B occupancy or histone H3 phosphorylation during metaphase (). These observations suggest that the effects of MAD2 reported here are only marginally influenced by MAD1 and thus soluble C-MAD2 is the likely entity that regulates the events downstream of the initial SAC activation.
Figure 4. MAD1 has a limited role in the regulation of AURORA B-mediated mitotic functions. (A). HeLa cells were transfected with control or MAD1 siRNA for 48 hours, followed by immunoblotting with anti-MAD1 and phosphorylation specific antibodies against histone H3 residues serine 10 and serine 28. Western blotting with histone H3 and TUBULIN antibodies were performed as controls. (B). Immunofluorescence analysis of control and MAD1 siRNA transfected HeLa cells was performed with the indicated antibodies to visualize Aurora B localization and the phosphorylation status of histone H3 S10 and S28 residues during metaphase. DNA was stained with Hoechst. Scale bar is 10 microns.
Discussion
The fidelity of chromosome segregation during cell division is controlled by the delicate balance of expression and activity of several spindle and mitotic checkpoint proteins.Citation16 Loss of MAD2 or other mitotic checkpoint components promotes catastrophic events such as early mitotic exit with chromosome mis-segregation and accumulation of aneuploidy. Activation of checkpoint signaling arrests the cells at metaphase until all the attachment errors are rectified and the chromosomes are properly bi-oriented at the metaphase plate. Together with these checkpoint proteins, the expression of checkpoint-regulatory factors such as p31 cometCitation17,18 and recently identified, WT1Citation7 modulate the signaling pathway via MAD2 interaction and regulate the timing of mitotic exit.
In this study we have demonstrated that silencing of MAD2 has effects beyond spindle/mitotic checkpoint function that are critical for the function of AURORA B kinase, one of the key members of CPC. MAD2 is important for the proper localization of AURORA B and also AURORA B-dependent histone H3 phosphorylation during mitosis. How MAD2 connects with AURORA B is not clear. Our data could be explained either by direct regulation of AURORA B catalytic activity, control of AURORA B localization, or a combination of these effects. The results presented here suggest that the regulation of AURORA B by MAD2 is likely to be independent of the MCC, but whether other intermediary factors are involved will require further studies. Our findings add a new dimension to the role of MAD2 in cell division where it regulates AURORA B recruitment and mitotic phosphorylation of several key residues of histone H3 (T3, S10 and S28). However, cell type specific differences may further fine-tune the effect of MAD2 on AURORA B function as evident from our results with MCF7 cells. Interestingly, an earlier studyCitation6 also reported contrasting effects in the non-cancerous cell line RPE1. It is therefore likely that additional cellular factors play an important role in MAD2-mediated regulation of AURORA B. In summary, our results suggest that MAD2 acts as a junction in connecting multiple cell cycle checkpoint signaling pathways (SAC, MCC and CPC) to maintain genomic stability.
Materials and methods
Cell culture and transfection
HeLa, WiT49 and MCF7 cells were grown in DMEM supplemented with 10% (vol/vol) FBS at 37°C. Transfection of plasmids was performed using Effectene reagent (Qiagen). Cells were harvested 48 hours after transfection and processed for different assays. MAD2, MAD1 and AURORA B siRNAs were obtained from Qiagen. siRNAs were transfected using Hiperfect reagent (Qiagen) for 48 hours. For the MAD2 rescue experiment siRNA targeting 3′UTR of MAD2 (CTGAAAGTAACTCATAATCTA) was used which has no affect on the coding sequence of the MAD2 overexpression plasmid.
Western blotting and immunofluorescence analysis
Western blotting analysis was performed as described beforeCitation7 using antibodies: MAD2 (C-19-sc-6329) and SURVIVIN (D-8, sc-17779) were obtained from Santa Cruz Biotechnology. MAD2 (A300-301A) and MAD1 (A300-339A) antibodies were obtained from Bethyl laboratories. H3S10p (ab5176), H3S28p (ab5169), H3 (ab1791), AURORA B (ab3609) and β-TUBULIN (ab6046) antibodies were obtained from Abcam. H3T3p (07–424) antibody was obtained from Millipore. Immunofluorescence analysis was performed as previously described.Citation7
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Files
Download MS Word (637.9 KB)Acknowledgments
We are grateful to Dr. K Kitagawa for providing human MAD2 clone. We thank Alan Siegel and UB North Campus Imaging Facility funded by NSF-MRI Grant DBI 0923133 for the confocal images.
Funding
This work was funded by the National Institute of General Medical Sciences (1R01GM098609 to K.F.M. and S.G.E.R.). The authors declare no competing financial interests.
References
- Schuyler SC, Wu YF, Kuan VJ. The Mad1-Mad2 balancing act–a damaged spindle checkpoint in chromosome instability and cancer. J Cell Sci 2012; 125:4197-206; PMID:23093575; http://dx.doi.org/10.1242/jcs.107037
- Musacchio A. The Molecular Biology of Spindle Assembly Checkpoint Signaling Dynamics. Curr Biol 2015; 25:R1002-18; PMID:26485365; http://dx.doi.org/10.1016/j.cub.2015.08.051
- Shandilya J, Roberts SG. A role of WT1 in cell division and genomic stability. Cell Cycle 2015; 14:1358-64; PMID:25789599; http://dx.doi.org/10.1080/15384101.2015.1021525
- Kitagawa M, Lee SH. The chromosomal passenger complex (CPC) as a key orchestrator of orderly mitotic exit and cytokinesis. Fron Cell Dev Biol 2015; 3:14; PMID:25798441; http://dx.doi.org/10.3389/fcell.2015.00014
- Krenn V, Musacchio A. The Aurora B Kinase in Chromosome Bi-Orientation and Spindle Checkpoint Signaling. Front Oncol 2015; 5:225; PMID:26528436; http://dx.doi.org/10.3389/fonc.2015.00225
- Kabeche L, Compton DA. Checkpoint-independent stabilization of kinetochore-microtubule attachments by Mad2 in human cells. Curr Biol 2012; 22:638-44; PMID:22405866; http://dx.doi.org/10.1016/j.cub.2012.02.030
- Shandilya J, Toska E, Richard DJ, Medler KF, Roberts SG. WT1 interacts with MAD2 and regulates mitotic checkpoint function. Nat Commun 2014; 5:4903; PMID:25232865; http://dx.doi.org/10.1038/ncomms5903
- Sawicka A, Seiser C. Histone H3 phosphorylation - a versatile chromatin modification for different occasions. Biochimie 2012; 94:2193-201; PMID:22564826; http://dx.doi.org/10.1016/j.biochi.2012.04.018
- Jeong YS, Cho S, Park JS, Ko Y, Kang YK. Phosphorylation of serine-10 of histone H3 shields modified lysine-9 selectively during mitosis. Gen Cells 2010; 15:181-92; http://dx.doi.org/10.1111/j.1365-2443.2009.01375.x
- Bian Y, Kitagawa R, Bansal PK, Fujii Y, Stepanov A, Kitagawa K. Synthetic genetic array screen identifies PP2A as a therapeutic target in Mad2-overexpressing tumors. Proc Natl Acad Sci U S A 2014; 111:1628-33; PMID:24425774; http://dx.doi.org/10.1073/pnas.1315588111
- Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, Gorbsky GJ, Higgins JM. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science 2010; 330:231-5; PMID:20705812; http://dx.doi.org/10.1126/science.1189435
- Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 2010; 330:235-9; PMID:20705815; http://dx.doi.org/10.1126/science.1189505
- Wang F, Ulyanova NP, van der Waal MS, Patnaik D, Lens SM, Higgins JM. A positive feedback loop involving Haspin and Aurora B promotes CPC accumulation at centromeres in mitosis. Curr Biol 2011; 21:1061-9; PMID:21658950; http://dx.doi.org/10.1016/j.cub.2011.05.016
- Zhou L, Tian X, Zhu C, Wang F, Higgins JM. Polo-like kinase-1 triggers histone phosphorylation by Haspin in mitosis. EMBO Rep 2014; 15:273-81; PMID:24413556
- van der Horst A, Lens SM. Cell division: control of the chromosomal passenger complex in time and space. Chromosoma 2014; 123:25-42; PMID:24091645; http://dx.doi.org/10.1007/s00412-013-0437-6
- Sacristan C, Kops GJ. Joined at the hip: kinetochores, microtubules, and spindle assembly checkpoint signaling. Trends Cell Biol 2015; 25:21-8; PMID:25220181; http://dx.doi.org/10.1016/j.tcb.2014.08.006
- Yang M, Li B, Tomchick DR, Machius M, Rizo J, Yu H, Luo X. p31comet blocks Mad2 activation through structural mimicry. Cell 2007; 131:744-55; PMID:18022368; http://dx.doi.org/10.1016/j.cell.2007.08.048
- Westhorpe FG, Tighe A, Lara-Gonzalez P, Taylor SS. p31comet-mediated extraction of Mad2 from the MCC promotes efficient mitotic exit. J Cell Sci 2011; 124:3905-16; PMID:22100920; http://dx.doi.org/10.1242/jcs.093286
