Control of eukaryotic cell division and growth involves molecular circuits, known as “cell cycle checkpoints” that stop or slow the cell cycle in conditions unfavorable for cell division and therefore ensure proper timing of cellular events. In response to appropriate cues, cells may either enter the cell cycle or persist in a quiescent (G0) or quiescent-like state. Historically, it has been well established that the DNA damage checkpoint and the spindle checkpoint are 2 essential signaling pathways which ensure proper division of the cell. Nevertheless, cells that make the decision to enter the cell cycle should be metabolically prepared to support the energetic and metabolic demand imposed by proliferation. It has become increasingly evident that metabolic pathways are integrated and coupled to cell cycle progression.Citation1 However, less is known about the mechanisms involved. In this brief feature editorial we highlight several key results that led us to define “the metabolic stress-induced cell cycle checkpoint,” which represents such interconnections and provides molecular mechanisms that sense cellular metabolic status and, in turn, regulates cell cycle, differentiation, and cellular function.
As one of the most ancient functional properties of cells, metabolism plays a critical role in determining the fate of diverse types of cells. In the last few years, there is increasing interest in uncovering the role of metabolism in the cell cycle, but analysis of this pathway in stem cells is still only in its infancy. Stem cells harbor a unique combination of several key properties, including maintenance of an undifferentiated state, self-renewal, and pluripotency to produce multiple cell types. Stem cells reversibly enter and exit the cell cycle over time, producing new stem cells (self-renewal) as well as various lineage-committed progenitors (differentiation). Generally, a stem cell needs to assess its energetic and metabolic status to ensure it can synthesize sufficient biomass to produce a new daughter cell, so it seems logical to propose that stem cells would significantly alter their metabolism as they transition from quiescence to an activated state. It is well known that haematopoietic stem cells (HSCs) are usually in a quiescent state and contain low numbers of mitochondria. Quiescent HSCs depend more on glycolysis for ATP synthesis than on mitochondrial oxidative phosphorylation (OXPHOS).Citation2 However, when HSCs enter a cell division program associated with differentiation, a robust energy demand is expected for this high energy/metabolism demand process. Indeed, ATP content is significantly increased in lineage-committed progenitors compared to that in HSCs.Citation3 Thus, stem cells and progenitor cells have distinct metabolic states, and the transition from stem to progenitor cells corresponds to a critical metabolic switch, namely one from glycolysis to OXPHOS. These observations underscore the importance of mitochondrial metabolism in HSCs. However, solid evidence for the mitochondrial metabolic regulation of HSC activities is still lacking.
By focusing on PTPMT1, a PTEN-like mitochondrial phosphatase that dephosphorylates phosphatidylinositol phosphatases (PIPs) in the mitochondrial inner membrane,Citation4,5 we have recently found that mitochondrial metabolism plays a critically important role for the differentiation of embryonic stem cells (ESCs)Citation5 and HSCs.Citation6 Depletion of PTPMT1 from ESCs and HSCs impairs their differentiation, resulting in peri-implantation embryonic lethality of global knockout mice and haematopoietic failure in conditional knockout mice, respectively. This is because mitochondrial aerobic metabolism is decreased following PTPMT1 depletion although cytosolic glycolysis is concomitantly enhanced. Strikingly, the HSC population is expanded by ∼40 and 25-fold in the percentage and absolute number, respectively, due to the complete inability of PTPMT1-dificient HSCs to undergo differentiation.Citation6
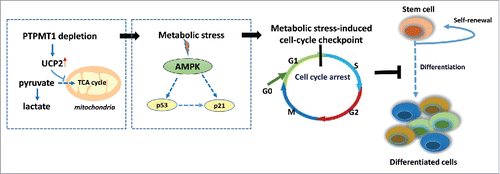
How do the mitochondrial metabolic defects lead to a differentiation block in stem cells? What is the link? PTPMT1 depletion does not seem to affect the ultra-structures of the mitochondria or cell survival. Rather, accumulation of PIP substrates due to the lack of dephosphorylation by PTPMT1 enhances the activity of uncoupling protein 2 (UCP2),Citation6 which is known to regulate cellular bioenergetics/metabolism by facilitating cytosolic glycolysis and decreasing mitochondrial metabolism. Consequently, pyruvate, a major substrate of the mitochondria that is derived from glucose, is shunted from the mitochondria to cytosolic glycolysis, resulting in mitochondrial metabolic stress, although cytosolic glycolysis is enhanced. AMP-activated kinase (AMPK), an intracellular metabolic stress sensor, is highly activated in PTPMT1-depleted stem cells. Activation of AMPK can induce a cell cycle arrest by phosphorylating and stabilizing the tumor suppressor gene p53.Citation7 Indeed, PTPMT1-deficient HSCs are delayed at the G1 phase in the cell cycle,Citation6 which is accompanied by the cellular accumulation of p53 and subsequent upregulation of p21 and p57, cyclin-dependent kinase inhibitors of the G1-to-S transition. These data support the idea that the mitochondrial metabolic stress caused by PTPMT1 deficiency is sensed by AMPK and this signaling is relayed to the cell cycle machinery through the p53–p21/p57 axis, leading to a cell cycle arrest in a subset of PTPMT1 knockout HSCs (). However, given the heterogeneity of stem cells and that the overall stem cell pool is drastically expanded in the knockout mice, it remains unclear how stem cell self-renewal and differentiation-associated divisions are differentially impacted by the metabolic/energetic stress. Notably, myeloid, T lymphoid, or B lymphoid lineage-specific PTPMT1 knockout mice do not show any defects in lineage cell development,Citation6 despite similar metabolic stress in the lineage progenitor lacking PTPMT1. These observations strongly suggest that the mitochondrial metabolic stress-triggered cell cycle checkpoint functions only in stem cells, but not lineage progenitors.
Figure 1. The metabolic stress-induced cell cycle checkpoint in PTPMT1-depleted HSCs. PTPMT1 deficiency enhances the activity of mitochondrial uncoupling protein 2 (UCP2), which inhibits pyruvate oxidation in the mitochondria, and thus impairs the metabolic shift from glycolysis to OXPHOS that is required for HSC differentiation. This metabolic stress activates AMPK, which in turn turns on the G1/S cell cycle checkpoint, leading to cell-cycle arrest in a subset of HSCs and a differentiation block.
Despite the emerging evidence for the influence of metabolic stress in the cell cycle progression, metabolic stress-induced cell cycle checkpoints and stress response pathways have, on the whole, been still poorly defined. Importantly, the relationship between metabolism and cell cycle progression may not be confined to normal stem cells. It seems likely that there is an important mechanistic link between metabolic alterations and the cell cycle effectors in cancer stem cells (CSCs), cancer precursor cells that are responsible for generation of bulk cancer cells, drug resistance, and metastasis. Does a similar metabolic stress-induced cell cycle checkpoint also operate in these malignant stem cells? Do CSCs have changes in their metabolic wiring that would make them distinct from normal stem cells and that could be harnessed for therapeutic intervention? Further studies should address these questions. An attractive future perspective of this field of research is to discover new therapeutic targets to control the functions of CSCs by inducing a cell cycle arrest through manipulating cellular metabolism.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Lee IH, Finkel T. Metabolic regulation of the cell cycle. Curr Opin Cell Boil 2013; 25:724-9; http://dx.doi.org/10.1016/j.ceb.2013.07.002
- Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 2011; 9:298-310; PMID:21982230; http://dx.doi.org/10.1016/j.stem.2011.09.010
- Inoue S, Noda S, Kashima K, Nakada K, Hayashi J-I, Miyoshi H. Mitochondrial respiration defects modulate differentiation but not proliferation of hematopoietic stem and progenitor cells. FEBS Lett 2010; 584:3402-9; PMID:20600007; http://dx.doi.org/10.1016/j.febslet.2010.06.036
- Pagliarini DJ, Worby CA, Dixon JE. A PTEN-like phosphatase with a novel substrate specificity. J Biol Chem 2004; 279:38590-6; PMID:15247229; http://dx.doi.org/10.1074/jbc.M404959200
- Shen J, Liu X, Yu W-M, Liu J, Nibbelink MG, Guo C, Finkel T, Qu C-K. A critical role of mitochondrial phosphatase Ptpmt1 in Embryogenesis Reveals a mitochondrial metabolic stress-induced differentiation checkpoint in embryonic stem cells. Mol Cell Biol 2011; 31:4902-16; PMID:21986498; http://dx.doi.org/10.1128/MCB.05629-11
- Yu WM, Liu X, Shen J, Jovanovic O, Pohl EE, Gerson SL, Finkel T, Broxmeyer HE, Qu C-K. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell 2013; 12:62-74; PMID:23290137; http://dx.doi.org/10.1016/j.stem.2012.11.022
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Bimbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 2005; 18:283-93; PMID:15866171; http://dx.doi.org/10.1016/j.molcel.2005.03.027
