Cells control the timing of their divisions by changing the length of each cell cycle phase. One notable role of such regulations is cell size control. The constant cell size of yeasts is maintained by the coupling of the cell cycle with growth.Citation1 Budding and fission yeasts achieve this coordination by triggering the G1/S or G2/M transition upon reaching a critical cell size, respectively. Thus their cell cycles are tightly coupled with growth. In multicellular organisms, however, it is known that cell cycles can be uncoupled from cell growth. This notion was clearly demonstrated in the fly wing disc.Citation2 Interestingly, the shortening of the G1 phase does not change the total cell cycle length because of the compensatory lengthening of the G2 phase and vice versa.Citation2 This phenomenon, called as “cell cycle compensation” is thought to be crucial for uncoupling the cell cycle from growth in multicellular organisms. Cell cycle compensation in the fly relies on mutual negative feedbacks between G1/S and G2/M CDKs; these feedbacks are mediated by the key transcription factor E2F, which promotes both G1/S and G2/M transitions.Citation2 Since the early stages of animal embryogenesis typically proceed without growth, it was not extensively examined whether similar compensatory relationships exist between different cell cycle phases during embryogenesis. On the other hand, it has been suggested that the timing of cell division during development shows exquisite spatiotemporal patterns that are coordinated with the morphogenetic cell movement. This regulation is necessary because the morphogenetic cell shape changes require cytoskeletal reorganizations that are incompatible with cell division.Citation3 Given that cell cycle compensation provides an opportunity for cells to achieve more complex cell cycle regulations compared to the respective regulations of different cell cycle phases, it is possible that a similar compensatory mechanism is exploited during the morphogenesis of early embryos.
Using the chordate ascidian as a model, we have recently discovered that the compensation between the S and G2 phases is exploited to regulate the mitotic pattern of epidermis during neural tube closure.Citation4 The first finding of our study is the S-phase dependency of the epidermal mitotic patterns. The cell cycles of the ascidian embryo are composed mostly of S, G2, and M phases, as are the cell cycles of other early animal embryos. In the fly embryo, the regulation of the G2 phase length is thought to be responsible for the timing of cell division. However, recent careful examinations using the fluorescent probes, which can discriminate the S-phase from the G2 phase, revealed the contribution of S phase length to the timing of cell division.Citation5 Our study employed a similar strategy to distinguish between the contributions of the S and G2 phases to the spatiotemporal pattern of mitosis. In ascidian, the neural plate folds up into a neural tube in the posterior-to-anterior directed manner, and this morphogenetic wave is immediately followed by a wave of a single round of epidermal mitosis with the same directionality (). By discriminating the S phase from the G2 phase of epidermal cells during this morphogenetic wave, we revealed that the timing of cell division is proportional to the S phase length but not to the G2 phase length, suggesting that S phase length is responsible for the pattern of mitosis. Interestingly, a recent study indicated that the differential S phase length controls the mitotic pattern in ascidian blastula embryos.Citation6 It is possible that S phase−dependent regulation of the mitotic pattern is a universal feature of early ascidian embryogenesis.
Figure 1. Role of cell cycle compensation in the switch from synchronous to patterned mitosis. The variation of S and G2 phase length along the anterior−posterior axis is schematically shown with the pattern of mitotic wave and morphogenesis, both of which are represented by arrows.
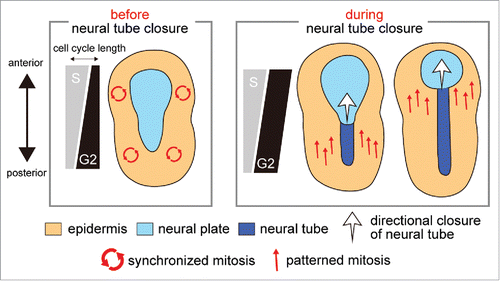
Our second key finding is that the G2 phase length compensates for the asynchronous S phase length to achieve the synchrony of epidermal mitosis before neural tube closure, which is required for proper closure. The compensatory G2 phase is suddenly lost during neural tube closure, thereby allowing cells to perform cell division in the patterned manner according to the asynchronous S phase length (). Our third finding is the identification of molecules responsible for the regulation of the G2 phase length. The compensatory regulation of the epidermal G2 phase is dependent on the asymmetric expression of the G2 phase regulator, cdc25 along the anterior–posterior axis. The asymmetry of cdc25 expression is conferred by the key transcription factors for the specification of epidermis, thus demonstrating the tight link between the regulation of cell cycle compensation and the developmental mechanism.
In this study, we could not address how the asynchronous S phase length is regulated; the mechanism underlying that regulation is the main target of our future study. Cell cycle compensation in the fly is based on the negative feedbacks between the regulation of the G1 and G2 phases.Citation2 We are interested in learning whether similar negative feedbacks exist between the regulations of the S and G2 phases of ascidian epidermal cells. It is likely that a shared molecule is the upstream factor that regulates both the S and G2 phases of epidermis in order to achieve their well-coordinated regulation. An attractive possibility is that the compensation between S and G2 phases is mediated by the antagonism of retinoic acid and FGF signalings, which is a conserved mechanism of anterior–posterior patterning in chordates including ascidianCitation7.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Turner JJ, Ewald JC, Skotheim JM. Cell size control in yeast. Curr Biol 2012; 22:R350-9; PMID: 22575477; http://dx.doi.org/10.1016/j.cub.2012.02.041
- Reis T, Edgar BA. Negative regulation of dE2F1 by cyclin-dependent kinases controls cell cycle timing. Cell 2004; 117:253-4; PMID: 15084262; http://dx.doi.org/10.1016/S0092-8674(04)00247-8
- Duncan T, Su TT. Embryogenesis: Coordinating cell division with gastrulation. Curr Biol 2004; 14:R305-7; PMID: 15084299; http://dx.doi.org/10.1016/j.cub.2004.03.050
- Ogura Y, Sasakura Y. Developmental control of cell-cycle compensation provides a switch for patterned mitosis at the onset of chordate neurulation. Dev Cell 2016; 37:148-61; PMID: 27093084; http://dx.doi.org/10.1016/j.devcel.2016.03.013
- McCleland ML, Shermoen AW, O'Farrell PH. DNA replication times the cell cycle and contributes to the mid-blastula transition in Drosophila embryos. J Cell Biol 2009; 187:7-14; PMID: 19786576; http://dx.doi.org/10.1083/jcb.200906191
- Dumollard R, Hebras C, Besnardeau L, McDougall A. Beta-catenin patterns the cell cycle during maternal-to-zygotic transition in urochordate embryos. Dev Biol 2013; 384:331-42; PMID: 24140189; http://dx.doi.org/10.1016/j.ydbio.2013.10.007
- Pasini A, Manenti R, Rothbächer U, Lemaire P. Antagonizing retinoic acid and FGF/MAPK pathways control posterior body patterning in the invertebrate chordate ciona intestinalis. PLoS One 2012; 7:e46193; PMID: 23049976; http://dx.doi.org/10.1371/journal.pone.0046193
