ABSTRACT
Sertoli cells (SCs) play a crucial role in testis differentiation, development and function, determining the magnitude of sperm production in sexually mature animals. For over 40 years, it has been considered that these key testis somatic cells stop dividing during early pre-pubertal phase, between around 10 to 20 days after birth respectively in mice and rats, being after that under physiological conditions a stable and terminally differentiated population. However, evidences from the literature are challenging this dogma. In the present study, using several important functional markers (Ki-67, BrdU, p27, GATA-4, Androgen Receptor), we investigated the SC differentiation status in 36 days old and adult Wistar rats, focusing mainly in the transition region (TR) between the seminiferous tubules (ST) and the rete testis. Our results showed that SCs in TR remain undifferentiated for a longer period and, although at a lesser degree, even in adult rats proliferating SCs were observed in this region. Therefore, these findings suggest that, different from the other ST regions investigated, SCs residing in the TR exhibit a distinct functional phenotype. These undifferentiated SCs may compose a subpopulation of SC progenitors that reside in a specific microenvironment capable of growing the ST length if needed from this particular testis region. Moreover, our findings demonstrate an important aspect of testis function in mammals and opens new venues for other experimental approaches to the investigation of SC physiology, spermatogenesis progression and testis growth. Besides that, the TR may represent an important site for pathophysiological investigations and cellular interactions in the testis.
Introduction
Sertoli cells (SCs) play a crucial role in testis differentiation, development, and function, particularly on the aspects related to spermatogenesis.Citation1-3 Furthermore, SCs are one of the key elements of the spermatogonial stem cells microenvironment (niche), providing the former with all the necessary substances regulating their proliferation and differentiation.Citation4-8
The number of SCs in the testis established before puberty determines the magnitude of sperm production in sexually mature animals.Citation2,9-11 In this regard, it is well known that SCs in laboratory rodents proliferate during the fetal and early postnatal period of testis development.Citation12-14 For over 40 years, the accepted paradigm has been that SCs cease to divide and become an adult, terminally differentiated cell population.Citation3,15,16 However, suggesting that SCs may still proliferate in some areas of the testis, even after the stabilization of the tubular diameter and SC efficiency (number of germ cells per SC) in early puberty, the testis continues to grow, with significant increases in weigh, sperm production and tubular length during the post-puberty period in several mammalian species investigated.Citation16-24 Although challenging the dogma that SCs do not proliferate in pre-pubertal and sexually mature mammals has been controversial, some mammalian seasonal breeders have been reported to show season-dependent variations in SC proliferation activity.Citation24-26
In all vertebrates spermatogenesis occurs in the seminiferous tubules (ST).Citation27 Particularly in mammals, the region that connects the seminiferous tubules to the rete testis is known as the transition region or transitional zone (TR)Citation28,29 and very few studies have been devoted to this particular area of the testis.Citation30-34 Anatomically, it is considered that this region is composed of morphologically modified SCs that form a plug-like valve structure on the luminal aspect of the seminiferous tubules.Citation33,35 Furthermore, few advanced germ cells are present in the TR,Citation30,31 and according to a recently published study,Citation36 this region emerges as a potential site or niche for spermatogonial stem cells. In their study, the stable and selective maintenance of Asingle GFRα1-positive spermatogonia was observed in the TR, as well as the expression of high levels of GDNF (the GFRα1 ligand), a major niche factor produced by SCs.Citation36
In order to better understand SC proliferation/differentiation dynamics in pre-pubertal and adult Wistar rats, in the present study we investigated several important factors related to this key testis somatic cell function, particularly in the TR (). Our findings suggest a distinct behavior/function of a SCs subset in this region.
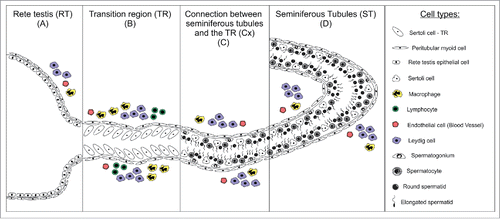
Figure 1. Schematic illustration of the different testicular parenchyma areas investigated in pre-pubertal and adult Wistar rats. The morphological and functional characteristics of Sertoli cells in the transition region (TR) (B), in the area adjacent to the transition region (Cx) (C), and along the other areas of seminiferous tubules (ST) (D) were evaluated. The Cx area was arbitrarily defined as a region of the ST corresponding to approximately 250 micrometers from the beginning of TR. Each cell type represented in the above scheme is depicted in the box located at the right side.
Results
Sertoli cell proliferation markers
As shown in , proliferative SCs were found only in TR in both pre-pubertal and adult rats. The analysis for Ki-67 revealed that approximately 4% of SCs were proliferating in this region in pre-pubertal rats on day 36. Although still proliferating, this activity was significantly reduced (less than 1%; p < 0.05) in adult rats. The above pattern was qualitatively confirmed by BrdU immunolabeling ().
Figure 2. Ki-67 immunostaining in different testicular areas of pre-pubertal (A-D) and adult (E-H) Wistar rats. Images showing the seminiferous tubules (ST; B and F), the area adjacent to the transition region (Cx; C and G) and the transition region (TR; D and H). Positive and negative Sertoli cells are indicated respectively by red and green arrowheads. In TR, Sertoli cells were observed proliferating at 36 and 120 days. The number of proliferating Sertoli cells observed in adults (∼1%) are significantly lower than those found in pre-pubertal rats (∼4%) (p < 0.05) (I). As expected, proliferating germ cells were observed in the seminiferous epithelium (yellow arrows). Bar: 50 μm (A and E); 10 μm (B-D, F-H).
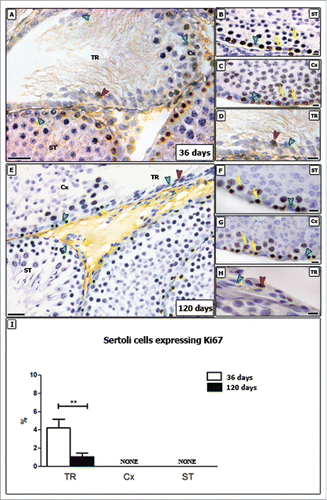
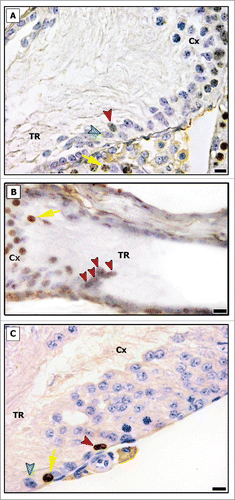
Figure 3. BrdU immunostaining in the transition region (TR) and in the area adjacent to the TR (Cx) in pre-pubertal (A and B) and adult (C) Wistar rats. Proliferating Sertoli cells were found in TR (red arrowheads), and a cluster of BrdU labeled Sertoli cells was found in pre-pubertal rat (B). Negative Sertoli cells are indicated by green arrowhead (A and C). Proliferating germ cells are shown by yellow arrows. Bar: 10 μm.
Sertoli cell differentiation markers
1P27
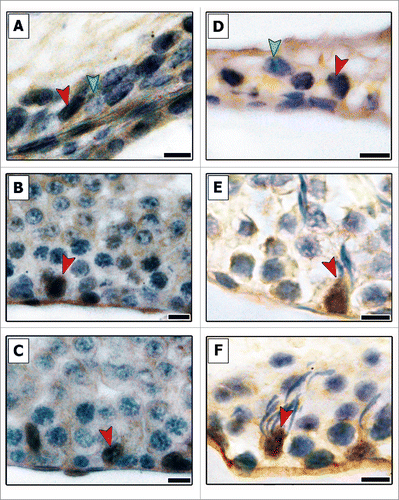
In contrast to the proliferative markers, p27 is a protein that promotes cell-cycle inhibition and is characteristically expressed in non-proliferative cells. Immunostaining for p27 showed an opposite pattern (), as p27-negative SCs were found only in the TR in both pre-pubertal and adult ages. The vast majority of SCs observed was p27-positive.
Figure 4. Evaluation of p27 immunostaining in different areas of testicular parenchyma in pre-pubertal (A–C) and adult (D–F) Wistar rats. The transition region (TR) (A and D), the area adjacent to the transition region (B and E) and along the seminiferous tubules (C and F) were investigated for this cell-cycle inhibitor. All Sertoli cells present in Cx and ST were positive for p27 (red arrowheads, B–F), whereas negative Sertoli cells (green arrowheads) were observed only in the TR. Bars: 10 μm.
GATA-4
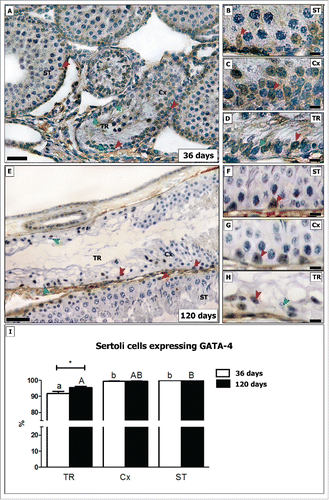
In both ages investigated, SCs not expressing the transcription factor GATA-4 were found mainly in the TR (). Approximately 8% of the GATA-4 negative SCs were found in pre-pubertal rats, while in adults this figure was significantly reduced (∼4%; p < 0.05). In the Cx (connection between seminiferous tubules and transition region; please see section 4.3), very few GATA-4 negative SCs were noted (less than 1%) in young and in adult rats. All SCs in the other tubular areas expressed GATA-4 ().
Figure 5. Evaluation of GATA-4 immunostaining in different testicular areas of pre-pubertal (A–D) and adult (E–H) Wistar rats. Images showing the seminiferous tubules (ST; B and F), the area adjacent to the transition region (Cx; C and G) and the transition region (TR; D and H). Positive and negative Sertoli cells are indicated respectively by red and green arrowheads. In TR, about 8% of Sertoli cells do not express GATA-4 in pre-pubertal rats. In adults, the percentage of GATA-4 negative Sertoli cells are reduced by half (4%; p < 0.05) (I). In the Cx (B, G), few GATA-4 negative Sertoli cells were found in both young and adult rats (less than 1%). Bar: 50 μm (A and E); 10 μm (B-D, F-H). Different small and capital letters represent statistically significant differences between regions (TR, ST or Cx) respectively of young and adult rats (p < 0.05). Considering the same region, statistically significant differences (p < 0.05) were observed only for TR.
Androgen receptor (AR)
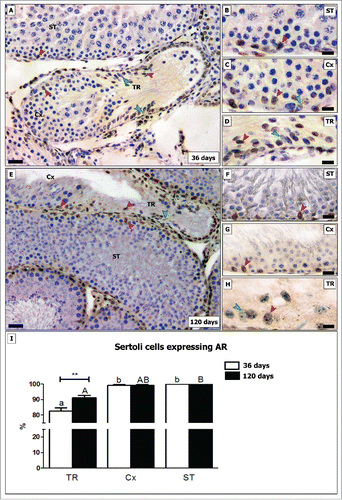
AR expression in SC is associated with the onset of puberty, but first appears postnatally after all major SC proliferation has begun to decline significantly.Citation37 AR-negative SCs located in the TR in pre-pubertal rats were observed frequently, as approximately one fifth (∼17%) of the SCs did not express AR. In adult rats its expression was halved (around 8%; p < 0.05) (). Regarding the Cx, very few negative SCs (less than 1%) were observed in both investigated ages. As expected, all SCs expressed AR in the other seminiferous tubular areas ().
Figure 6. Evaluation of AR immunostaining in different testicular areas of pre-pubertal (A-D) and adult (E-H) Wistar rats. Images showing the seminiferous tubules (ST; B and F), the area adjacent to the transition region (Cx; C and G) and the transition region (TR; D and H). Positive and negative Sertoli cells are indicated respectively by red and green arrowheads. In TR, approximately 17% of Sertoli cells do not express AR in pre-pubertal rats. In adults, the percentage of AR negative Sertoli cells are reduced by half (8%; p < 0.05) (I). In the Cx (B, G), few AR negative Sertoli cells were observed in both young and adult rats (less than 1%). Bar: 50 μm (A and E); 10 μm (B-D, F-H). Different small and capital letters represent statistically significant differences between regions (TR, ST or Cx) respectively for young and adult rats (p < 0.05). Considering the same region, statistically significant differences (p < 0.05) were observed only for TR.
Immunofluorescence
Double-staining for Ki-67 and AR in the 3 regions showed a distinct immunolabeling pattern in both pre-pubertal and adult rats, as all Ki-67-positive SCs were AR-negative (). A particularly interesting pattern was exhibited by some peritubular myoid cells in the TR and epithelial cells from the rete testis, which were positive for the proliferation marker Ki-67 as well as for AR ().
Figure 7. Double-label immunofluorescence for Ki-67 (A, D) and AR (B, E) in pre-pubertal Wistar rats. As it can be observed, AR positive Sertoli cells are Ki-67 negative (orange arrow C, F), while Ki-67 positive Sertoli cells are AR negative (white arrowhead C, F). Peritubular myoid cells were observed in TR expressing Ki-67 and AR (blue arrow C). TR: transition region; Cx: area adjacent to the TR; ST: seminiferous tubules. Bar: 50 μm.
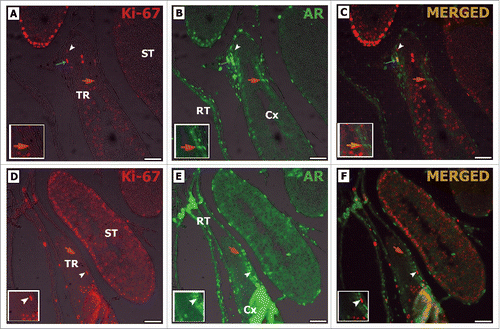
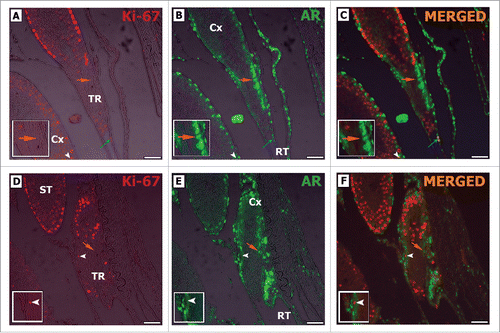
Figure 8. Double-label immunofluorescence for Ki-67 (A, D) and AR (B, E) in adult Wistar rats. As it can be noted, AR positive Sertoli cells are Ki-67 negative (orange arrow C, F), while Ki-67 positive Sertoli cells are AR negative (white arrowhead C, F). Rete testis epithelial cells were also observed expressing Ki-67 and AR (RT blue arrow C). TR: transition region; Cx: area adjacent to the TR; ST: seminiferous tubules. Bar: 50 μm.
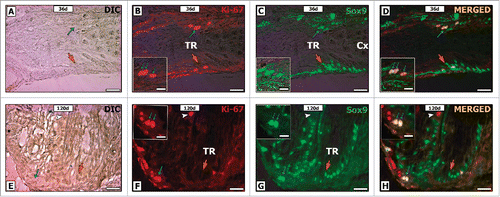
Double-staining for Ki-67 and Sox-9 confirmed that the somatic cells located in the TR epithelium were positive for both markers (). Considering their nuclear morphology, location and Sox-9 expression, these somatic cells might be considered as putative Sertoli cells.
Figure 9. Double-label immunofluorescence for Ki-67 (B, F) and Sox-9 (C, G) in pre-pubertal (A-D) and adult (E-H) Wistar rats. As it can be seen, some Sox-9 positive Sertoli cells was also Ki-67 positive (blue arrow D, H). Otherwise, other Sox-9 positive Sertoli cells were negative for Ki-67 (orange arrow D, H). Few proliferative germ cells were also observed in the TR (white arrowhead H). TR: transition region; Cx: area adjacent to the TR. Bar: 50 μm.
Discussion
This study is the first to report that Sertoli cell proliferation in a rodent species can occur well beyond the perinatal period and even into pre-pubertal and adult life. For over 40 years, it has been reported that Sertoli cell proliferation in the laboratory rodent peaks just prior to birth and that SCs stop dividing before puberty, between 15-21 days of age in the rat.Citation12,13,38-40 The potential for SC proliferation beyond the perinatal period was thought to occur only under specific experimental conditions.Citation24-26,41,42 Nevertheless, it is well known that the testis increases in size well into adulthood, with a significant increase in total lengths of the seminiferous tubules, but without a further increase in tubular diameter and SC efficiency.Citation19,43 However, a satisfactory explanation for this tubular lengthening has not been presented until now. Immature Sox-9 positive SCs, capable of proliferation, were found in the transition region of the seminiferous tubules, adjacent to the rete testis. These cells would permit a slow but continuous growth of the tubules, as well as the preservation of progenitor SCs that are capable of extending the period of mitotic activity.
Prior studies of SC proliferation appear to have overlooked the rete testis junction, as they did not comment on the transition region.Citation12,13,38 Therefore, it was reasonable for those studies to conclude that SCs no longer divide after postnatal day 21. Other observations also supported this traditional view. In the rat, SC differentiation begins between 14 and 21 days postnatal and is marked by at least 3 key morphological events: an intense proliferation of primary spermatocytes, formation of the blood-testis barrier (SCs barrier) and the opening of the seminiferous tubule lumen due to SC secretions.Citation14,44 Our results also found that SCs outside the transition region were positive only for differentiation markers and at 36 and 120 days of age, only within the rete testis connection did SCs exhibit markers for proliferation.
A small portion of SCs within the transition region displayed features of being undifferentiated and negative for p27, GATA-4 and AR. GATA-4 staining of the SC nucleus is an accepted marker for the differentiating SC.Citation45,46 This transcription factor is mainly expressed in Sertoli and Leydig cells and has been implicated in the development and function of the mammalian testis, particularly in the regulation of gene expression and cell differentiation.Citation47,48
In the current study, 5-10% of the SCs in the transition region did not express GATA-4 at both ages investigated, while in other regions of the testis, all SCs were positive for this factor. In the testis, GATA-4 regulates SC differentiation and function and is required for proper interaction between these somatic cells and germ cells.Citation45,49,50 In the conditional knockout of GATA-4, the testis was atrophic, with impairment of spermatogenesis and loss of fertility. Most importantly, SCs exhibited altered morphology and had increased permeability at the blood-testis barrier,Citation45 consistent with an immature phenotype. Dmrt1 is also under GATA-4 regulation and in the Dmrt1 knockout mouse, SCs failed to complete differentiation and exhibited over-proliferation.Citation51-53
AR is an inducible transcription factor that regulates gene expression in response to androgens,Citation54-56 and also serves as a marker for SC maturation.Citation37 An increase in AR expression in SC is associated with the general termination of SC proliferation.Citation57 In contrast, the absence of AR inhibits SC maturation.Citation58 A recent report found that hormonal suppression in men resulted in an increased SC expression of Ki-67 and PCNA, which was coincident with a decrease in AR, suggesting that SC are capable of de-differentiation.Citation24 In the current study, SCs within the transition region appear to remain undifferentiated from the perinatal period.
Thyroid hormones (TH) are considered the main regulators of SC proliferation and differentiation.Citation22,23,59-61 TH acts through the cell-cycle inhibitors p21 and p27,Citation14,62-64 and serves as an important regulator of AR, increasing its expression in the rat SC.Citation65 Thus, the presence of a pool of AR- and p27-negative SCs in the transition region also indicates a small pool of undifferentiated SCs reside in this unique region.
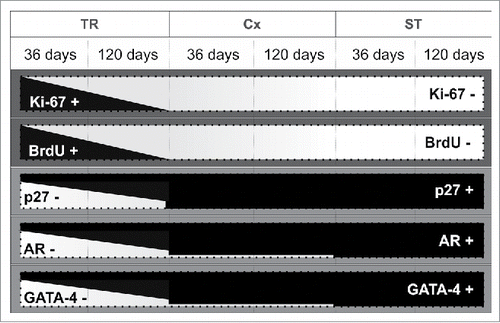
In conclusion, although it has been accepted for many years that SCs stop dividing by day 21 in the rodent species,Citation12,13,44 data obtained in the present study indicate that SCs at the seminiferous tubule/rete testis junction, known as the transition region, express markers for immature and proliferating cells at 36 and 120 days of age (). It is possible that this discovery has been delayed due to the fact that most rodent samples collected for histology are taken from transverse sections of the testis at the midline, often overlooking the rete testis region that is located off-center and more cephalic.Citation66 The current study highlights the need to include samples from the transition region when testing for potential effects on SC proliferation. Proliferation beyond the early postnatal period was thought to occur only under certain experimental conditions such as hemicastration,Citation67 transient hypothyroidism,Citation61 hormonal suppression,Citation24 SC transplantation,Citation68 and during recrudescence in hibernating mammals.Citation23,69 However, the data presented here suggests that SCs within the transition region exhibit a distinct functional phenotype, long after the differentiation of SCs located in other regions of the seminiferous tubules. These undifferentiated SCs may compose a subpopulation of SCs progenitors that are capable of growing the seminiferous tubules if necessary. In this context, several questions are raised. Is the transition region a potential site for chemical toxicity or the onset of specific diseases? Could these immature SCs be a site for antigen leakage into rete testis fluid? Do new germ cell clonal units fill new stem cell niches that would form after SC division?
Figure 10. Diagram summarizing the results found in the present study. Representation of positive (+; black areas) and negative (-; white areas) Sertoli cells for each functional marker considered (Ki67, BrdU, p27, AR, and GATA-4) in the 3 testis parenchyma regions evaluated in 36 and 120 days-old Wistar rats. As shown schematically, the relative expression of these markers in TR is clearly distinct when compared to the other 2 investigated regions (Cx and ST). TR: transition region; Cx: area adjacent to the TR; ST: seminiferous tubules.
Methods
Animals
Twenty Wistar rats (Rattus novergicus; aged 36 and 120 days) were used in this study. All animal experiments were performed in strict accordance with the Guidelines for Animal Use and Experimentation as set forth by the Animal Experimentation Ethics Committees from the Federal University of Minas Gerais (Belo Horizonte, Brazil; CEUA 398/2013).
Histology and immunostaining
Rats were sacrificed by pentobarbital overdose (50 mg/Kg BW). For immunohistochemical staining and according to the specific antibodies used, testes samples from 16 rats were fixed for 24h in Bouin's solution or 4% paraformaldehyde (PFA) or 10% formalin. The samples were then dehydrated in ethanol and routinely embedded in paraplast. Serial sections (5 μm thick) from the chosen area were incubated overnight at 4°C with the following antibodies and dilutions: anti-AR (androgen receptor; 1:50; Santa Cruz Biotechnology, sc-816), anti-GATA4 (1:100; Santa Cruz Biotechnology, sc-25310), anti-Ki-67 (1:100; PharMingen, #558615) or anti-p27 (1:50; PharMingen, #554069). Reactions were visualized using biotin-conjugated secondary antibodies in combination with the Elite ABC Kit (Vector Laboratories, CA) or using Alexa-488 anti-rabbit/633 anti-mouse conjugated secondary antibodies (1:200 dilution; Thermo Fisher Scientific) - visualized using a Nikon fluorescence microscope (Eclipse Ti). Aiming to simultaneously evaluate the SCs regarding their proliferative and differentiation status, a double-staining for Ki-67 and AR in pre-pubertal and adult rats was also performed. A double-staining for Ki-67 and Sox-9 (anti-Sox-9; 1:50 dilution; Santa Cruz Biotechnology, sc-20095) was also performed to demonstrate that Sertoli cells are indeed able to proliferate in the TR. Additionally, in order to investigate qualitatively SC proliferation activity focusing exclusively on the S phase of the cell cycle (Ki-67 protein is present during all active phases of the cell cycle; G1, S, G2, and mitosis), BrdU (150 mg/Kg BW) was intraperitoneally injected into 4 rats (2 pre-pubertals and 2 adults) 2h before their sacrifice, and this evaluation was performed using anti-BrdU (1:100 dilution; PharMingen, #347580).
Quantitative analyses
After immunolabeling following standardized protocols,Citation70,71 the evaluated samples were photographed at 200x and, according to the illustration shown in , the SC functional markers were quantified in the TR, in the connection between seminiferous tubules and the TR (Cx; arbitrarily defined as 250 μm of extension) and along the other ST areas. For Ki-67, AR and GATA4, the percentage of positive/negative SCs was calculated in each of the 3 investigated regions and, according to a pilot study and statistical analysis, at least an area of 50.000µm2 per region was evaluated. All the stained samples were analyzed using the image analysis software Image J v.1.45s (Image Processing and Analysis, in Java) and an Olympus microscope (BX60).
Statistical analyses
All data were tested for normality and homoscedasticity of the variances. Quantitative data are represented as the mean ± SEM (standard error of mean). Analyses were conducted using the graphics and statistics program PRISM v5.0 (GraphPad Software, Inc.). Data were assessed by one-way ANOVA for comparisons within groups followed by Newman-Keuls test in case of normal distribution, or by Kruskal-Wallis followed by Dunn's test in case of nonparametric data. Student's unpaired t-test was performed for single comparisons between groups. Differences were considered statistically significant at p < 0.05.
Abbreviations
| AR | = | Androgen receptor |
| Cx | = | Connection between seminiferous tubules and transition region |
| SC | = | Sertoli cell |
| ST | = | Seminiferous tubules |
| TR | = | Transition region |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Conceived and designed the experiments: AFAF, LRF, GMJC. Performed the experiments: AFAF, GMJC. Analyzed the data: AFAF, GMJC. Contributed reagents/materials/analysis tools: AFAF, GMJC. Wrote the paper: AFAF, LRF, RAH, GMJC.
Acknowledgments
Technical help from Mara Lívia Santos is highly appreciated.
Funding
The scholarships awarded to AFAF and the financial support from the PRPq-UFMG, FAPEMIG and CNPq are fully appreciated.
References
- Russell LD, Ettlin RA, Sinha-Hikim AP, Clegg ED. Histological and histopathological evaluation of the testis. 1st ed. Russel LD, Ettlin RA, Sinha-Hikim AP, technical editors. Clearwater: Cache River Press; 1990.
- Sharpe RM. The physiology of reproduction. 2nd ed. New York: Raven Press: 1994. Regulation of spermatogenesis; p.1363-1434
- França LR, Russell LD. Male reproduction: a multidisciplinary overview. 1st ed. Madrid: Churchill Livingstone; 1998. The testis of domestic animals; p.197-219.
- Oatley JM, Brinster RL. The germline stem cell niche unit in Mammalian testes. Physiol Rev 2012; 92(2):577-95; PMID:22535892
- Yoshida S. Elucidating the identity and behavior of spermatogenic stem cells in the mouse testis. Reproduction 2012; 144(3):293-302; PMID:22733803; http://dx.doi.org/10.1530/REP-11-0320
- Chen SR, Liu YX. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli. Reproduction 2015; 149(4):R159-67; PMID:25504872; http://dx.doi.org/10.1530/REP-14-0481
- Zakhidov ST, Marshak TL. Experimental evidence of proliferation and reproduction of highly differentiated Sertoli cells. Izv Akad Nauk Ser Biol 2015; 42(4):350-60; PMID:26415275
- França LR, Hess RA, Dufour JM, Hofmann MC, Griswold MD. The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology 2016; 4(2):189–212; PMID:26846984; http://dx.doi.org/10.1111/andr.12165
- Hess RA, Cooke PS, Bunick D, Kirby JD. Adult testicular enlargement induced by neonatal hypothyroidism is accompanied by increased Sertoli and germ cell numbers. Endocrinology 1993; 132(6):2607-13; PMID:8504761
- França LR, Ye S, Ying L, Sandberg M, Russell LD. Morphometry of rat germ cells during spermatogenesis. Anat Rec 1995; 241(2):181-204; PMID:7710135
- Hess RA, França LR. Molecular Mechanisms in Spermatogenesis. 1st ed. New York: Landes Bioscience; 2007. Spermatogenesis and Cycle of the Seminiferous Epithelium; p.1-15.
- Steinberger A, Steinberger E. Replication pattern of Sertoli cells in maturing rat testis in vivo and organ culture. Biol Reprod 1971; 4(1):84-7; PMID:5110903
- Orth JM. Proliferation of sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec 1982; 203(4):485-92; PMID:7137603
- Auharek AS, França LR. Postnatal testis development, Sertoli cell proliferation and number of different spermatogonial types in C57BL/6J mice made transiently hypo- and hyperthyroidic during the neonatal period. J Anat 2010; 216(5):577-88; PMID:20525087; http://dx.doi.org/10.1111/j.1469-7580.2010.01219.x
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003; 125(6):769-84; PMID:12773099
- Hayrabedyan S, Todorova K, Pashova S, Mollova M, Fernández N. Sertoli cell quiescence - new insights. Am J Reprod Immunol 2012; 68(6):451-5; PMID:22531009; http://dx.doi.org/10.1111/j.1600-0897.2012.01137.x
- Ekwall H, Jansson A, Sjöberg P, Plöen L. Differentiation of the rat testis between 20 and 120 days of age. Arch Androl 1984; 13(1):27-36; PMID:6534278
- Berndtson WE, Thompson TL. 1990 Changing relationships between testis size, Sertoli cell number and spermatogenesis in Sprague-Dawley rats. J Androl 1990; 11(5):429-35; PMID:2254176
- Castro ACS, Cardoso FM, França LR. Effects of puberty and sexual development on daily sperm production and epididymal sperm reserves on Piau boars. Anim Reprod Sci 25:83-90
- Meisami E, Sendera TJ, Clay LB. Paradoxical hypertrophy and plasticity of the testis in rats recovering from early thyroid deficiency: a growth study including effects of age and duration of hypothyroidism. J Endocrinol 1992; 135(3):495-505; PMID:1487702
- Russell LD. Pathobiology of the aging rat. 1st ed. Washington: ILSI Press; 1992. Normal development of the testis; p.395-405
- França LR, Silva-Jr VA, Chiarini-Garcia H, Garcia SK, Debeljuk L. Cell proliferation and hormonal changes during postnal development of the testis in the pig. Biol Reprod 2000; 63(6):1629-36; PMID:11090429
- Tarulli GA, Stanton PG, Meachem SJ. Is the adult Sertoli cell terminally differentiated? Biol Reprod 2012; 1:87(1): 13, 1-11; PMID:22492971; http://dx.doi.org/10.1095/biolreprod.111.095091
- Tarulli GA, Stanton PG, Meyts ER, Loveland KL, McLachlan RI, Meachem SJ. A survey of Sertoli cell differentiation in men after gonadotropin suppression and in testicular cancer. Spermatogenesis 2013; 1:3(1):e24014; PMID:23687617
- Johnson L, Thompson DL. Age-related and seasonal variation in the Sertoli cell population, daily sperm production and serum concentrations of follicle-stimulating hormone, luteinizing hormone and testosterone in stallions. Biol Reprod 1983; 29(3):777-89; PMID:6414546
- Johnson L, Varner DD, Tatum ME, Scrutchfield WL. Season but not age affects Sertoli cell number in adult stallions. Biol Reprod 1991; 45(3):404-10; PMID:1782288
- Schulz RW, França LR, Lareyre JJ, LeGac F, Chiarini-Garcia H, Nobrega RH, Miura T. Spermatogenesis in fish. Gen Comp Endocrinol 2010; 1:165(3):390-411; PMID:19348807; http://dx.doi.org/10.1016/j.ygcen.2009.02.013
- Perey B, Clermont Y, Lehlond CP. The wave of the seminiferous epithelium in the rat. Am J Anat 1961; 108:47-78
- Roosen-Runge EC. The rete testis in the albino rat: its structure, development and morphological significance. Acta Anat (Basel) 1961; 45:1-30; PMID:14493722
- Dym M. Spermatogonial stem cells of the testis. Proc Natl Acad Sci USA 1994; 22:91(24): 11287-9; PMID:7972051
- Nykänen M. Fine structure of the transitional zone of the rat seminiferous tubule. Cell Tissue Res 1979; 25:198(3):441-54; PMID:466681
- Osman DI. On the histochemistry of the seminiferous tubules and their terminal segments in the ram testis. Anat Histol Embryol 1984; 13(4):319-26; PMID:6240947
- Hermo L, Dworkin J. Transitional cells at the junction of seminiferous tubules with the rete testis of the rat: their fine structure, endocytic activity, and basement membrane. Am J Anat 1988; 181(2):111-31; PMID:3285659
- Takahashi K, Naito M, Terayama H, Qu N, Cheng L, Tainosho S, Itoh M. Immunomorphological aspects of the tubuli recti and the surrounding interstitium in normal mice. Int J Androl 2007; 30(1):21-7; PMID:17328721
- Russell LD. The Sertoli cell. 1st ed. Clearwater: Cache River Press; 1993. Form, dimensions, and citology of mammalian Sertoli cells; p.1-37
- Aiyama Y, Tsunekawa N, Kishi K, Kawasumi M, Suzuki H, Kanai-Azuma M, Kurohmaru M, Kanay Y. A niche for GFRα1-positive spermatogonia in the terminal segments of the seminiferous tubules in hamster testes. Stem Cells 2015; 33(9):2811-24; PMID:26013732; http://dx.doi.org/10.1002/stem.2065
- Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, Denolet E, Verhoeven G. The role of androgen in Sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of androgen receptor. Endocrinology 2005; 146(6):2674-83; PMID:15761038
- Clermont Y, Perey B. Quantitative study of the cell population of the seminiferous tubules in immature rats. Am J Anat 1957; 100(2):241-67; PMID:13435229
- Russell LD, Clermont Y. Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. Anat Rec 1977; 187(3):347-66; PMID:851237
- Matthiesson KL, McLachlan RI, O'Donnell L, Frydenberg M, Robertson DM, Stanton PG, Meachem SJ. The relative roles of follicle-stimulating hormone and luteinizing hormone in maintaining spermatogonial maturation and spermiation in normal men. J Clin Endocrinol Metab 2006; 91(10):3962-9; PMID:16895950
- Sridharan S, Simon L, Meling DD, Cyr DG, Gutstein DE, Fishman GI, Guillou F, Cooke PS. Proliferation of adult Sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin 43) in mice. Biol Reprod 2007; 76(5):804-12; PMID:17229929
- Ahmed EA, Rijbroek AV, Kal HB, Sadri-Ardekani H, Mizrak SC, Pelt A, De Rooij DG. Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol Reprod 2009; 80(6):1084-91; PMID:19164176; http://dx.doi.org/10.1095/biolreprod.108.071662
- Swierstra EE. Influence of breed, age, and ejaculation frequency on boars semen composition. Can J Anim Sci 1973; 53:43-53
- Russell LD, Bartke A, Goh JC. Postnatal development of the Sertoli cell barrier, tubular lumen, and cytoskeleton of Sertoli and myoid cells in the rat, and their relationship to tubular fluid secretion and flow. Am J Anat 1989; 184(3):179-89; PMID:2750675
- Kyrönlahti A, Euler R, Bielinska M, Schoeller EL, Moley KH, Toppari J, Heikinheimo M, Wilson DB. GATA4 regulates Sertoli cell function and fertility in adult male mice. Mol Cell Endocrinol 2011; 10:333(1):85-95; PMID:21172404; http://dx.doi.org/10.1016/j.mce.2010.12.019
- Yang JZ, Zhao YF, Wang YM, Jing CX, Miao NZ, Ai QY. Determination of GATA-4 in the testis of the mouse. Zhonghua Nan Ke Xue 2010; 16(10):901-4; PMID:21243753
- Oreal E, Mazaud S, Picard JY, Magre S, Carre-Eusebe D. Different patterns of anti-Mullerian hormone expression, as related to Dmrt1, SF-1, WT1, GATA-4, Wnt-4, and Lhx9 expression, in the chick differentiating gonads. Dev Dyn 2002; 225(3):221-32; PMID:12412004
- Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol 2008; 22(4):781-98; PMID:18174356; http://dx.doi.org/10.1210/me.2007-0513
- Bielinska M, Seehra A, Toppari J, Heikinheimo M, Wilson DB. GATA-4 is required for sex steroidogenic cell development in the fetal mouse. Dev Dyn 2007; 236(1):203-13; PMID:17096405
- Tevosian SG. Transgenic mouse models in the study of reproduction: insights into GATA protein function. Reproduction 2014; 148(1):R1-R14; PMID:24700325; http://dx.doi.org/10.1530/REP-14-0086
- Raymond CS, Murphy MW, O'sullivan G, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev 2000; 15:14(20):2587-95; PMID:11040213
- Lei N, Heckert LL. Gata4 regulates testis expression of Dmrt1. Mol Cell Biol 2004; 24(1):377-88; PMID:14673170
- Manuylov NL, Zhou B, Ma Q, Fox SC, Pu WT, Tevosian SG. Conditional ablation of Gata4 and Fog2 genes in mice reveals their distinct roles in mammalian sexual differentiation. Dev Biol 2011; 15:353(2):229-41; PMID:21385577; http://dx.doi.org/10.1016/j.ydbio.2011.02.032
- Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev 2002; 23(2):175-200; PMID:11943742
- O'Donnell L, Pratis K, Wagenfeld A, Gottwald U, Muller J, Leder G, McLachlan RI, Stanton PG. Transcriptional profiling of the hormone-responsive stages of spermatogenesis reveals cell-,stage-, and hormone-specifics events. Endocrinology 2009; 150(11):5074-84; PMID:19797402; http://dx.doi.org/10.1210/en.2009-0755
- Stanton PG, Sluka P, Foo CFH, Stephens AN, Smith AI, McLachlan RI, O'Donnell L. Proteomic changes in rat spermatogenesis in response to in vivo manipulation; impact on meiotic cells. PLoS One 2012; 7(7):e41718; PMID:22860010; http://dx.doi.org/10.1371/journal.pone.0041718
- Hazra R, Corcoran L, Robson M, McTavish KJ, Upton D, Handelsman DJ, Allan CM. Temporal role of Sertoli cell androgen receptor expression in spermatogenic development. Mol Endocrinol 2013; 27(1):12-24; PMID:23160479; http://dx.doi.org/10.1210/me.2012-1219
- Willems A, Batlouni SR, Esnal A, Swinnen JV, Saunders PTK, Sharpe RM, França LR, De Gendt K, Verhoeven G. Selective ablation of the androgen receptor in mouse Sertoli cells affects Sertoli cells maturation, barrier formation and cytoskeletal development. PLoS One 2010; 30:5(11):e14168; PMID:21152390; http://dx.doi.org/10.1371/journal.pone.0014168
- Orth JM. The role of follicle-stimulating hormone in controlling Sertoli cell proliferation in testes of fetal rats. Endocrinology 1984; 115(4):1248-55; PMID:6090096
- Cooke PS, Zhao YD, Bunick D. Tri-iodothyronine inhibits proliferation and stimulates differentiation of cultured neonatal Sertoli cells: possible mechanism for increased adult testis weight and sperm production induced by neonatal goitrogen treatment. Biol Reprod 1994; 51(5):1000-5; PMID:7531505
- Holsberger DR, Jirawatnotai S, Kiyokawa H, Cooke OS. Thyroid hormone regulates the cell cycle inhibitor p27Kip1 in postnatal murine Sertoli cells. Endocrinology 2003; 144(9):3732-8; PMID:12933641
- Van Haaster LH, De Jong FH, Docter R, De Rooij DG. High neonatal triiodothyronine levels reduce the period of Sertoli cell proliferation and accelerate tubular lumen formation in the rat testis, and increase serum inhibin levels. Endocrinology 1993; 133(2):755-60; PMID:8344214
- Cooke PS, Holsberger DR, França LR. The Sertoli Cell Biology. San Diego: Elsevier – Academic Press; 2005. Thyroid hormone regulation of Sertoli cell development; p.217-226
- Holsberger DR, Buchold GM, Leal MC, Kiesewetter SE, O'Brien DA, Hess RA, França LR, Kiyokawa H, Cooke PS. Cell-cycle inhibitors p27Kip1 and p21Cip1 regulate murine Sertoli cell proliferation. Biol Reprod 2005; 72(6):1429-36; PMID:15728790
- Arambepola NK, Bunick D, Cooke PS. Thyroid hormone effects on androgen receptor messenger RNA expression in rat Sertoli and peritubular cells. J Endocrinol 1998; 156(1):43-50; PMID:9496232
- Ilio KY, Hess RA. Structure and function of the ductuli efferentes: a review. Microsc Res Tech 1994; 15:29(6):432-67; PMID:7873793
- Lunstra DD, Wise TH, Ford JJ. Sertoli cells in the boar testis: changes during development and compensatory hypertrophy after hemicastration at different ages. Biol Reprod 2003; 68(1):140-50; PMID:12493706
- Mital P, Kaur G, Bowlin B, Paniagua NJ, Korbutt GS, Dufour JM. Biol Reprod 2014; 23:90(1):13; PMID:24285718; http://dx.doi.org/10.1095/biolreprod.113.110197
- Tarulli GA, Stanton PG, Lerchl A, Meachem SJ. Adult sertoli cells are not terminally differentiated in the Djungarian hamster: effect of FSH on proliferation and junction protein organization. Biol Reprod 2006; 74(5):798-806; PMID:16407497
- Giorno R. A comparison of two immunoperoxidase staining methods based on the avidin-biotin interaction. Diagn Immunol 1984; 2(3):161-6; PMID:6388981
- Ramos-Vara JÁ. Technical aspects of Immunohistochemistry. Vet Pathol 2005; 42(4):405-26; PMID:16006601
