ABSTRACT
We have previously discovered nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells and have shown that they can differentiate to neurons, glia, and many other cell types. HAP stem cells can be used for nerve and spinal cord repair. We have recently shown the HAP stem cells can differentiate to beating heart-muscle cells and tissue sheets of beating heart-muscle cells. In the present study, we determined the efficiency of HAP stem cells from mouse vibrissa hair follicles of various ages to differentiate to beating heart-muscle cells. We observed that the whiskers located near the ear were more efficient to differentiate to cardiac-muscle cells compared to whiskers located near the nose. Differentiation to cardiac-muscle cells from HAP stem cells in cultured whiskers in 4-week-old mice was significantly greater than in 10-, 20-, and 40-week-old mice. There was a strong decrease in differentiation potential of HAP stem cells to cardiac-muscle cells by 10 weeks of age. In contrast, the differentiation potential of HAP stem cells to other cell types did not decrease with age. The possibility of rejuvenation of HAP stem cells to differentiate at high efficiency to cardiac-muscle cells is discussed.
Introduction
We have previously demonstrated that the neural stem-cell marker nestin is expressed in hair follicle stem cells, located in the bulge area, which are termed hair-follicle-associated pluripotent (HAP) stem cells.Citation1 HAP stem cells from mouse and human could differentiate to neurons, glia, keratinocytes, smooth muscle cells, and melanocytes in vitro.Citation2 HAP stem cells also could effect nerve and spinal cord regeneration in mouse models.Citation3-6 We recently demonstrated that HAP stem cells differentiated to beating cardiac-muscle cells. The differentiation potential to cardiac-muscle is greatest in the upper part of the follicle. The beat rate of the cardiac-muscle cells was stimulated by isoproterenol and inhibited by propanolol.Citation7
We subsequently observed that isoproterenol directs HAP stem cells to differentiate to cardiac-muscle cells in large numbers. The addition of activin A, bone-morphogenetic protein 4, and basic fibroblast growth factor, along with isoproternal, induced the HAP-stem-cell-derived cardiac-muscle cells to form tissue sheets of beating heart-muscle cells.Citation8
In the present report, we demonstrate that early aging selectively reduces the ability of HAP stem cells to differentiate to cardiac-muscle cells.
Results and discussion
Vibrissa follicles from 4-week-old mice have a high potential for differentiation to cardiac-muscle cells
We determined whether the differentiation of vibrissa (whisker) follicles to cardiac-muscle cells was age dependent. We isolated vibrissa hair follicles from 4-, 10-, 20-, and 40-week-old mice. Vibrissa follicles located near the ear side were larger than those located near the nose side (). We cultured whisker follicles individually in 24-well cell-culture plates and aligned them in wells of the dish corresponding to their location in the mouse (). We observed beating heart-muscle cells differentiating from the whisker follicles at 4 weeks in culture. The experiments were repeated on 4 samples. The greatest percentage of whisker follicles differentiating to beating cardiac-muscle cells was from 4-week-old mice. The percentage of whisker follicles that produced beating heart-muscle cells per one whisker pad from 4-week-old mice was: 43.01 ± 9.86 %; 10-week-old: 13.98 ± 8.12 %; 20-week-old: 21.51 ± 13.04 %; 40-week-old: 12.90 ± 3.23 %) (). P-value of 4-week-old vs. 10-week old: = 0.008; P-value of 4-week-old vs. 20-week old: = 0.042; P-value of 4-week-old vs. 40-week old: = 0.004.
Figure 1. Whisker follicles from 4-week-old mice have greater potential for differentiation to cardiac-muscle cells than older mice. (A) Location map of mouse whisker follicles from one pad in one cheek. Blue box = nose side of the mouse whisker pad, Red box = ear side of the mouse whisker pad. (B) Whisker follicles from 4-week-old mice have a greater potential for differentiation to cardiac-muscle cells compared to 10-, 20-, and 40-week-old mice (n = 4 or each time point). (C) Comparison of the differentiation efficiency to cardiac-muscle cells from whisker follicles with mice of different age. (D) Anti-cTnT levels (ELISA analysis) in 4-, 10-, 20- and 40-week-old mouse cardiac-muscle cells differentiated from cultured whiskers. (E) Comparisons of whisker follicles from the ear side and nose side for differentiation potential to cardiac-muscle cells.
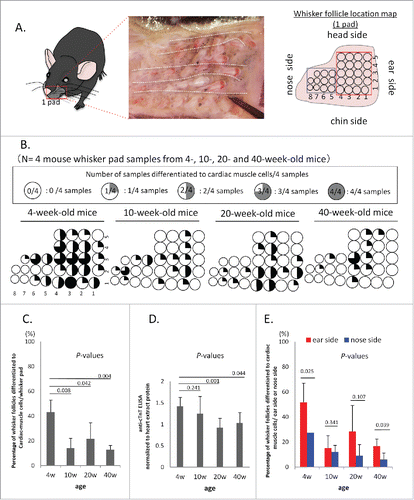
Cardiac troponin (cTnT) also had higher levels in cardiac-muscle cells differentiating from cultured whisker follicles from 4-week-old mice than from whisker follicles from 10-, 20-, and 40-week-old mice (4-weeks-old; vs. 10-week-old, P = 0.241; 4-week-old vs. 20-week-old, P = 0.001; 4-week-old vs. 40-week-old, P = 0.044) ().
We compared whisker follicles located in the ear side with whisker follicles located in the nose side for differentiation to beating cardiac-muscle cells. Whisker follicles located in the ear side differentiated to beating cardiac-muscle cells in greater amounts than those located in the nose side. A higher percentage of the whisker follicles produced beating cardiac-muscle cells in the ear side in mice of all ages tested: (4-week-old: ear side 51.7 ± 15.3%, nose side 27.3 ± 0 %; 10-week-old: ear side 15.0 ± 10%, nose side 12.1 ± 5.2%; 20-week-old: ear side 28.3 ± 20.8%, nose side 9.0 ± 9.0%; 40-week-old: ear side 16.7 ± 5.8%, nose side 6.0 ± 5.2% (). Ear side vs. nose side, 4-week-old, P = 0.025; ear side vs. nose side, 10-week-old, P = 0.341; ear side vs. nose side, 20-week-old, P = 0.107; ear side vs. nose side, 40-week-old, P = 0.039.
The greatest number of cells that differentiated to beating cardiac-muscle cells was from whisker follicles from 4-week-old mice: 4-week-old: 18 ± 0.58 × 105; 10-week-old: 6 ± 1.00 × 105; 20-week-old: 11 ± 2.50 × 105; 40-week-old: 10 ± 1.50 × 105 per whisker pad (). P-value of 4-week-old vs. 10-week-old, = 0.00003; P-value of 4-week-old vs. 20-week-old, = 0.003; P-value of 4-week-old, vs. 40-week-old, = 0.0003.
Figure 2. Differentiation to cardiac-muscle cells from whisker follicles from 4-, 10-, 20- and 40-week-old mice. (A) The total differentiated cardiac-muscle cell number from the upper part of whisker follicles from the ear side of the whisker pad, cultured in DMEM with 10% FBS. (B) Number of cTnT-positive cells differentiated from the whisker follicles from the ear side of the whisker pad, shown by flow cytometry. (C) Percentage of cTnT-positive cardiac-muscle cells differentiated from whisker follicles. (D) The number of cardiac-muscle cells differentiated from the upper part of whisker follicles.
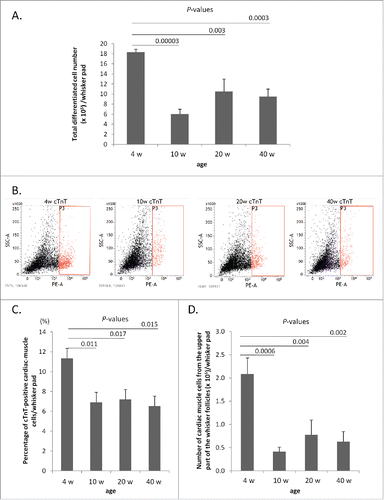
Flow cytometry analysis showed that cTnT-positive cells differentiating from whisker follicles from 4-week-old mice were greater than from 10-, 20-, and 40-week-old mice (). P-value of 4-week-old vs. 10-week-old, = 0.011; P-value of 4-week-old vs. 20-week-old, = 0.017; P-value of 4-week-old vs. 40-week-old, = 0.015.
The number of cardiac-muscle cells differentiated from the upper part of hair follicles from 4-week-old mice was significantly greater from 10-, 20-, and 40-week-old mice: 4-week-old: 2.08 ± 0.35 × 105; 10-week-old: 0.41 ± 0.10 × 105; 20-week-old: 0.78 ± 0.32 × 105; 40-week-old: 0.63 ± 0.22 × 105 (). P-value of 4-week-old vs. 10-week-old, = 0.0006; P-value of 4-week-old vs. 20-week-old, = 0.004; P-value of 4-week-old vs. 40-week-old, = 0.002.
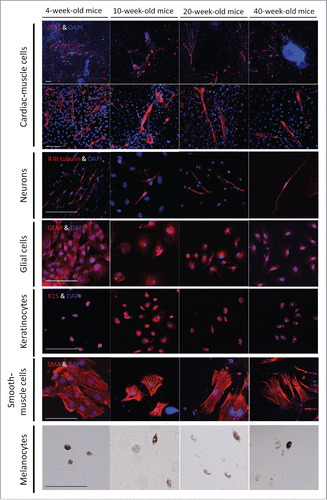
In order to determine whether the differentiation to neurons, glial cells, smooth muscle cells, keratinocytes, and melanocytes changed with age, the upper parts of hair follicles from 4,- 10-, 20-, and 40-week-old mice were cultured in Mattek culture plates. Immunofluorescence staining showed that hair follicle differentiation potential to other cell types was maintained with age ().
Figure 3. The differentiation potential to various cell types from whisker follicles from mice of different ages. Immunofluorescence staining showed that whisker follicle HAP stemcells from 4-, 10-, 20-, and 40-week-old mice differentiated to cardiac muscle cells, neurons, glial cells, keratinocytes and smooth muscle cells. Red = cTnT, tubulin, GFAP, Keratin 15 (K15) and smooth muscle actin (SMA). Blue = DAPI. Bar = 100 µm. Phase-contrast images showed that HAP stem cell from 4-, 10-, 20-, and 40-week-old mice also differentiated to melanocytes. Bar = 100 µm.
The mRNA level of stem-cell-marker genes does not show any significant difference with age
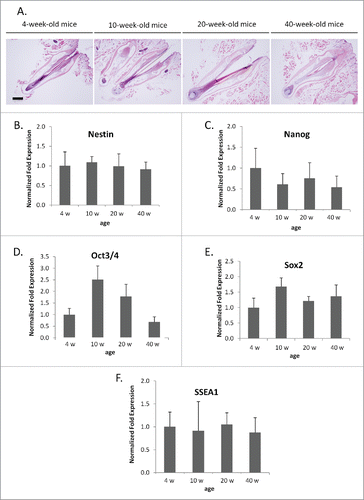
We also measured the expression level of stem cell markers with age including nestin, Nanog, Oct 3/4, Sox 2, and SSEA1 in the upper parts of whisker follicles from 4-, 10-, 20-, and 40-week-old mice using real-time RT-PCR. Expression of GAPDH in whiskers of 4-week-old mice was used as a control. None of these stem-cell makers showed a change in expression in the whisker follicle with age () (P > 0.05).
Figure 4. mRNA levels of stem cell marker genes from whisker hair follicles of various age. (A) Hematoxylin and eosin staining of whisker follicles isolated from 4-, 10-, 20-, and 40-week-old mice. Bar = 200 µm. (B-F) Comparisons of mRNA levels of stem-cell-marker genes: nestin (B), Nanog (C), Oct 3/4 (D), Sox 2 (E), SSEA1 (F) were determined with PCR. Results are mean ± SD for 3 samples each. The mRNA level of stem-cell-marker genes did not show any significant differences with whisker age.
Nishimura et al. showed that hair graying is caused by defective self-maintenance of melanocyte stem cells in aging mice.Citation9 Cheng et al.Citation10 showed induced pluripotent stem (iPS) cells from bone marrow-derived myeloid cells from aged mice had lower differentiation efficiency than iPS cells from young mice. Horibe et al.Citation11 showed that human mobilized dental-pulp stem cells (MDPSCs) from aged donors decreased in differentiation potential, migration, and proliferation compared to MDPSCs from young donors. We have recently shown that transplanted hair follicles decreased their hair-growth potential with age and HAP stem cells changed their distribution in the aged follicles. However, senescence of hair follicles could be reversed.Citation12 Perhaps the age-related reduction in HAP stem cell ability to differentiate to beating heart-muscle cells can also be rejuvenated.
We have recently shown that HAP stem cells and whole whisker follicles can be cryopreserved.Citation9,10 HAP stem cells show great promise for regenerative medicine, since they differentiate to many useful cell types, are easily accessible, non-tumorigenic, and are not immunogenic, because they are available from each patient. Since the HAP stem cells decrease, their potential for differentiation to beating heart-muscle cells at an early age, and hair follicles become senescent with age, banking hair follicles at an early age may be beneficial to every person for potential future use.
Previous studies indicated that stem cells may be able to be rejuvenated such as MTOR inhibitors, such as rapamycin,Citation11 or modulators of TP53 by nutlin-3a.Citation12
Future experiments will determine if such treatments can rejuvenate the ability of aging hair follicles to more efficiently differentiate to cardiac-muscle cells.
Materials and methods
Mice
C57BL/6J mice; 4-, 10-, 20- and 40-week-old mice, were obtained from CLEA (Kawasaki, Japan) and used to isolate whisker hair follicles. All animal experiments were conducted according to the Guidelines for Animal Experimentation at Kitasato University.
Whisker follicles and culture
The whisker follicles from mice were isolated as described previously.Citation3 The upper parts of whisker follicles were seeded onto BD Matrigel™ (basement-membrane matrix growth-factor reduced) (BD Biosciences, Bedford, MA), and cultured in DMEM (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (FBS), 50 µg/ml gentamycin (GIBCO, Grand Island, NY), 2 mM L-glutamine (GIBCO) and 10 mM HEPES (MP Biomedicals, Santa Ana, CA). Beating cardiac-muscle cells were recorded by video microscopy (ScopPad-500, GelleX, Tokyo, Japan).
Anti–cardiac-troponin T (cTnT) ELISA analysis of differentiated cells from the upper parts of whisker follicles
The upper parts of mouse whisker follicles were cultured in 24-well cell plates (Corning, Kennebunk, ME) for 4-weeks. The cells were fixed and incubated with anti-cardiac-troponin T (cTnT) mouse monoclonal antibody (1:2000, Gene Tex, Hsinchu City, Taiwan) for 1 hour. The cells were then incubated with HRP-protein A/G Purified Recomb® (1:10000, Thermo scientific, Rockford, IL) for 1 hour. The whisker follicles were removed from the well and the remaining cells were incubated with TMB+Substrate-Chromogen (Dako, Carpintaria, CA) for 15 minutes. H2SO4 1 N was then added to each well. The supernatant was transferred to 96-well EIA/RIA plates (Corning, Corning, NY) and measured with a Multi-Functional Microplate Reader SPECTRA FLUOR Plus (Wako, Tokyo, Japan).
Real-time polymerase chain reaction (RT-PCR) of stem cell marker genes
Total RNA from 20 upper parts of hair follicles was purified with RNeasy® Plus Mini Kit (QIAGEN, Hilden, Germany) and treated with the OneStep™PCR Inhibitor-Removal Kit (ZYMO RESEARCH, Irvin, CA) to remove melanin. c-DNA was synthesized with the SuperScript®VILO™ cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). Real-time PCR, on CFX96 (Bio-Rad, Hercules, CA) with TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) and TaqMan Probes, was performed as follows: GAPDH: Mm99999915_g1; Nanog: Mm02019550_s1; nestin: Mm00450205_ml; Oct 3/4: Mm03053917_g1; Sox 2: Mm03053810_s1; and SSEA1: Mm00487448_s1. The mRNA levels were normalized with GAPDH. Results are mean ± SD for 3 samples each.
Immunofluorescence staining of differentiated cells from the upper part of whisker follicles
The upper part of whisker follicles was cultured in Matteck plates (MatTeK, Ashland, MA) for 4-weeks. The cells were incubated with anti–cardiac-troponin T (cTnT) mouse monoclonal antibody (1:250, Gene Tex, Hsinchu City, Taiwan); anti-ß III tubulin mouse monoclonal antibody (1:250, Tuj1 clone, Covance, CA); anti-glial fibrillary acidic protein (GFAP) chicken polyclonal antibody (1:250; abcam, UK); anti–smooth-muscle actin (SMA) mouse monoclonal antibody (1:250; Lab Vision, Fremont, CA); and anti-keratin 15 (K15) mouse monoclonal antibody (1:250; Lab Vision, UK) in blocking buffer (4 % BSA, 0.5 % Triton X100, 0.04 % NaN3 in PBS) at room temperature for 3 hours. The cells were then incubated with goat anti-mouse IgG conjugated with Alexa Flour 568® (1:400, Molecular Probes, Eugene, Oregon) or goat anti-chicken IgG conjugated with Alexa Flour 568® (1:400, Molecular Probes, Eugene, Oregon) and 4′, 6-diamino-2-phenylindole, dihydrochloride (DAPI) (Molecular Probes) in blocking buffer for 2h at room temperature. Fluorescence staining was visualized using a laser-scanning microscope (710) (Carl Zeiss, Oberkochen, Germany).
cTnT flow cytometry of differentiated cells from the upper part of whisker follicles
The upper part of hair follicles was cultured in 6-well flat bottom plates (Corning, Kennebunk, ME) for 4-weeks. The cells were incubated with anti-cTnT mouse monoclonal antibody (1:200) as the primary antibody. The secondary antibody was goat anti-mouse IgG H&L phycoerythrin (1:500; Abcam, Cambridge, UK). The cells were measured with a FACS Verse (BD Bioscience) and were analyzed with FACS suite™ software (BD Bioscience). Flow-cytometry results are presented as mean ± SD for 3 samples each.
Statistical analysis
The experimental data are expressed as the mean ± SD. Statistical analyses were performed with the unpaired Student's t-test with a P-value of < 0.05 considered significant.
Dedication
This paper is dedicated to the memory of A. R. Moossa, MD.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary files
Download Zip (21.1 MB)Funding
This work was partially supported by a Grant-in-Aid for Scientific Research (C) 23591653 from the Ministry of Education, Science, Sports, and Culture of Japan, a grant from the Ministry of Education, Culture, Sports, Science, and Technology of the Japan Government (Assistance for Strategic Creation of Research Basis, 2014–2016), and the Terumo Life-Science Foundation (to Y. Amoh) and National Institute of Neurological Disorders and Stroke grant NS086217 to AntiCancer Inc.
References
- Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman RM. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci USA 2003; 100:9958-61; PMID:12904579; http://dx.doi.org/10.1073/pnas.1733025100
- Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin negative hair-follicle-bulge stem cells can form neurons. Proc Natl Acad Sci USA 2005; 102:5530-4; PMID:15802470; http://dx.doi.org/10.1073/pnas.0501263102
- Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, Hoffman RM. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci USA 2005; 102:17734–8; PMID:16314569.
- Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 2008; 7:1865–9; PMID:18583926.
- Liu F, Uchugonova A, Kimura H, Zhang C, Zhao M, Zhang L, Koenig K, Duong J, Aki R, Saito N, Mii S, Amoh Y, Katsuoka K, Hoffman RM. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle 2011; 10:830–9; PMID:21330787.
- Amoh Y, Kanoh M, Niiyama S, Hamada Y, Kawahara K, Sato Y, Hoffman RM, Katsuoka K. Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: An advantageous alternative to ES and iPS cells. J Cell Biochem 2009; 107:1016–20; PMID:19507228.
- Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Hoffman RM, Amoh Y. From hair to heart: nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells differentiate to beating cardiac-muscle cells. Cell Cycle 2015; 14:2362-6; PMID:25970547; http://dx.doi.org/10.1080/15384101.2015.1042633
- Yamazaki A, Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Hoffman RM, Amoh Y. Isoproterenol directs hair follicle-associated pluripotent (HAP) stem cells to differentiate in vitro to cardiac-muscle cells which can be induced to form beating heart muscle tissue sheets. Cell Cycle 2016; 15:760-5; PMID:27104748; http://dx.doi.org/10.1080/15384101.2016.1146837
- Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 2005; 307:720-4; PMID:15618488; http://dx.doi.org/10.1126/science.1099593
- Cheng Z, Ito S, Nishio N, Xiao H, Zhang R, Suzuki H, Okawa Y, Murohara T, Isobe K. Establishment of induced pluripotent stem cells from aged mice using bone marrow-derived myeloid cells. J Mol Cell Biol 2011; 3:91-8; PMID:21228011; http://dx.doi.org/10.1093/jmcb/mjq044
- Horibe H, Nakashima M, Iohara K, Hayashi Y, Takeuchi N, Takei Y, Kurita K, Nakashima M. Isolation of a stable subpopulation of mobilized dental pulp stem cells (MDPSCs) with high proliferation, migration, and regeneration potential is independent of age. PLoS One 2014; 9:e98553; PMID:24870376; http://dx.doi.org/10.1371/journal.pone.0098553
- Cao W, Li L, Kajiura S, Amoh Y, Tan Y, Liu F, Hoffman RM. Aging hair follicles rejuvenated by transplantation to a young subcutaneous environment. Cell Cycle 2016; 15(8):1093-8; PMID:26940664; http://dx.doi.org/10.1080/15384101.2016.1156269
- Kajiura S, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Li L, Katsuoka K, Hoffman RM, Amoh Y. Cryopreservation of the hair follicle maintains pluripotency of nestin-expressing hair follicle-associated pluripotent stem cells. Tissue Eng Part C 2015; 21:825-31; http://dx.doi.org/10.1089/ten.tec.2014.0500
- Cao W, Li L, Tran B, Kajiura S, Amoh Y, Liu F, Hoffman RM. Extensive hair shaft growth after mouse whisker follicle isolation, cryopreservation and transplantation in nude mice. PLoS One 2015; 10:e0145997; PMID:26716690; http://dx.doi.org/10.1371/journal.pone.0145997
- Leontieva OV, Gudkov AV, Blagosklonny MV. Weak p53 permits senescence during cell cycle arrest. Cell Cycle 2010; 9:4323-7; PMID:21051933; http://dx.doi.org/10.4161/cc.9.21.13584
- Blagosklonny MV. Aging, stem cells, and mammalian target of rapamycin: a prospect of pharmacologic rejuvenation of aging stem cells. Rejuvenation Res 2008; 11:801-8; PMID:18729812; http://dx.doi.org/10.1089/rej.2008.0722
