ABSTRACT
Mesenchymal stromal cells (MSCs) are a heterogeneous population, which contain several cell phenotypes: mesenchymal stem cells, progenitor cells, fibroblasts and other type of cells. Previously, we identified unique stem cells that we named multilineage-differentiating stress enduring (Muse) cells as one to several percent of MSCs of the bone marrow, adipose tissue and dermis. Among different cell populations in MSCs, Muse cells, positive for pluripotent surface marker SSEA-3, may represent cells responsible for pluripotent-like property of MSCs, since they express pluripotency genes, able to differentiated into triploblastic cells from a single cells and are self-renewable.
MSCs release biologically active factors that have profound effects on local cellular dynamics. A thorough examination of MSC secretome seems essential for understanding the physiological functions exerted by these cells in our organism and also for rational cellular therapy design. In this setting, studies on secretome of Muse cells may shed light on pathways that are associated with their specific features.
Our findings evidenced that secretomes of MSCs and Muse cells contain factors that regulate extracellular matrix remodeling, ox-redox activities and immune system. Muse cells appear to secrete factors that may preserve their stem cell features, allow survival under stress conditions and may contribute to their immunomodulation capacity.
In detail, the proteins belonging to protein kinase A signaling, FXR/RXR activation and LXR/RXR activation pathways may play a role in regulation of Muse stem cell features. These last 2 pathways together with proteins associated with antigen presentation pathway and coagulation system may play a role in immunomodulation.
Introduction
Mesenchymal stromal cells (MSCs) are a heterogeneous population, which contains several cell phenotypes: mesenchymal stem cells, progenitor cells, fibroblasts and other stromal cells. Besides mesenchymal stem cells, which can differentiate into mesodermal derivatives (adipocytes, chondrocytes and osteocytes), MSCs were suggested to contain pluripotent-like cells. This because MSC cultures showed differentiation not only into the same mesodermal-lineage they belong but also into ectodermal-lineage, such as neural and epidermal cells, as well as into endodermal-lineage, such as hepatocytes and β cells.Citation1-6 However, efficiency of differentiation is usually not high, suggesting that this phenomenon is dependent on a small subpopulation.Citation7
Previously, we identified unique stem cells that we named multilineage-differentiating stress enduring (Muse) cells as one to several percent of the total MSCs of the bone marrow, adipose tissue and dermis.Citation8 Among different cell populations in MSCs, Muse cells, which can be identified as cells positive for pluripotent surface marker SSEA-3, were initially found as stress tolerant cells, and show several aspects that may represent pluripotent-like differentiation ability of MSCs.Citation7,9 They are recognized to express pluripotent genes, able to differentiated into triploblastic cells from a single cells and are self-renewable, suggesting their pluripotency. In contrast, cells other than Muse cells in MSCs do not show either of these characters.Citation7,9 For example, when fibroblasts were separated into Muse and non-Muse cells and were subjected to melanocyte induction, Muse cells were converted to melanin-producing functional melanocytes but non-Muse cells responded partially to the induction and failed to become melanocytes.Citation10 This suggested that Muse cells are able to cross oligolineage boundary between mesodermal to ectodermal because they are pluripotent but non-Muse cells could not because they do not have pluripotent-like property. Other than differentiation ability, they are reported to exert tissue repair effect by replenishment of lost cell types by spontaneous differentiation after homing into damaged site; they spontaneously differentiated into dermal and epidermal cells to repair skin ulcer of diabetes mellitus model.Citation11 In stroke model, they spontaneously differentiated into neuronal cells and participated in reconstruction of pyramidal tract, and in hepatectomy model, intravenously injected Muse cells differentiated into liver components after homing.Citation12,13 Since naïve Muse cells were used in all these studies, Muse cells do not need to be induced into purposive cells prior to transplantation, unlike embryonic stem (ES) and induced pluripotent stem (iPS) cells. These unique performances are expected to be useful for regenerative medicine, while other functional aspect of Muse cells remains to be clarified.
It has been acknowledged that one of the main tasks accomplished by MSCs, both in physiological and clinical settings (cell transplant procedures), is to release several paracrine signals responsible for chemoattractant, immunomodulatory, angiogenic, anti-apoptotic, anti-scarring, and pro-survival effects.Citation14-17
Many findings aimed at identifying only a subset of factors released by MSCs at high levels, such as proteins involved in immune system signaling (i.e., IL-6, IL-8, MCP-1, and TGF-ß), extracellular matrix remodelers (i.e., TIMP-2, fibronectin, lumican, periostin, collagen, decorin, and metalloproteinase inhibitors), growth factors and their regulators (i.e., VEGF, GM-CSF, BMP2, bFGF, IGFBP3, IGFBP4, and IGFBP7). Only recently a more systematic and integrated approach for analysis of the MSC secretome that includes LC-MS/MS detection, antibody arrays, microarrays, and bioinformatic analysis has been employed.Citation7,18,19
We decided to perform an unbiased LC-MS/MS analysis of secretomes of whole MSC populations and compare these with Muse cell secretome in order to identify factors that may be associated with specific features of Muse cells. To carry out a comprehensive comparative analysis, we selected whole MSC populations from bone marrow and adipose tissue (bmMSCs and aMSCs, respectively), which share several biological features but also demonstrate peculiarities in their differentiation potential, transcriptome, proteome, and immunomodulation function.Citation20
Materials and methods
MSC cultures
Bone marrow was obtained from healthy donors who provided informed consent. We separated cells on a Ficoll density gradient (GE Healthcare, Italy), and the mononuclear cell fraction was collected and washed in phosphate-buffered saline (PBS). We seeded 1–2.5 × 105 cells/cm2 in α-MEM containing 10% fetal bovine serum (FBS) and bFGF. After 72 hours, non-adherent cells were discarded and adherent cells were cultivated to confluency. These cells (passage 0) were further amplified to conduct experiments at passages 2–3.
Lipoaspirates were obtained from healthy donors undergoing plastic surgery after they provided their informed consent. The dispersion of adipose tissue was achieved via collagenase digestion, after which the lipid-filled adipocytes' ability to float caused them to separate from the stromal vascular fraction by way of centrifugation. Stromal pellets were washed with PBS and further purified on a density gradient (Histopaque, GE Healthcare, UK). Mononuclear cells fractions were collected and cultivated in in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS. These cells (passage 0) were further amplified to conduct experiments at passages 2–3.
Collection of Muse cells
Bone marrow MSCs were cultured in low-glucose DMEM containing 10% FBS, 1 ng/mL bFGF, 2 mM GlutaMAX (ThermoFisher Scientific, Japan) and kanamycin, and were sub-cultured for 4 times. Confluent cells were collected by 0.25% trypsin-EDTA, and were subjected for cell sorting to isolate Muse cells, as described previously.Citation8 In brief, cells were suspended in FACS Buffer, which contained 0.5% bovine serum albumin (BSA), 2 mM EDTA-2H2O in FluoroBrite DMEM (ThermoFisher Scientific, Japan) and were incubated with anti-human SSEA-3 antibody (1:400, BioLegend, Japan) for one hour on ice. Cells were then washed with FACS buffer for 3 times and centrifuged at 400 g for 5 min. Subsequently, cells were incubated with secondary antibody, anti-Rat IgM-FITC (1:100, Jackson ImmunoResearch, PA, USA) for one hour on ice, and then washed 3 times again. SSEA-3(+) cells were sorted by FACSAria II Cell Sorter (Becton Dickinson, UK) using FITC filter. A low stream speed was used to ensure a high level of cell survival. Collected Muse cells were cultured in 10% FBS, 1 ng/mL bFGF, 2 mM GlutaMAX, kanamycin in low-glucose DMEM for over night at 37°C 5% CO2 and then subjected to analysis.
CM preparation for LC-MS/MS analysis
Without disturbing the attached cells, 5 mL of secretomes were collected from culture dishes and culture debris removed by centrifugation at 10,000 g. Supernatants were used for protein pooling with resin (StrataClean, Agilent Technology, CA, USA) using dried beads mixed with 1× Laemmli gel loading buffer and run on a gradient gel 4–15% SDS-PAGE (Criterion TGX Stain-Free Precast Gels, Bio-Rad, CA, USA). Following electrophoresis at 100 V, the gels were stained with Coomassie brilliant blue and gel lanes of interest excised for in-gel digestion, as previously described.Citation21
After digestion, peptides were eluted from the gel matrix by immersing the reaction tube in an ultrasonic bath for 5 min with a sequential elution of 0.4% formic acid in 3% ACN, 0.4% formic acid in 50% ACN, and 100% ACN. The supernatant containing the peptides was centrifuged, transferred to low binding tubes, and desalted by using pipette tips (ZipTip C18, Merck Millipore, Germany). Following that, extracted peptides were dried and stored at −80°C until LC-MS/MS analysis was performed. A more detailed protocol of CM preparation appears in Supplementary File 8.
LC-MS/MS analysis
Tandem mass spectrometric analysis was carried out using AB SCIEX TripleTOF 5600+ instrument (AB SCIEX, Redwood City, CA, USA) coupled to Eksigent expert nano-LC 400 system (AB SCIEX). MS and MS/MS data was acquired using Analyst® TF v.1.6 (AB SCIEX).
Mass spectrometry data was analyzed by using ProteinPilot 4.5 Beta (AB SCIEX) for the peptide identifications. Detailed protocol in supplementary file 8.
GO and network analyses
Proteins expressed in secretomes were analyzed with PANTHER (http://www.pantherdb.org) and IPA (http//www.ingenuity.com/product/ipa).
Using PANTHER, protein classification was performed according to 3 ontological terms: biological processes, molecular functions, and molecular classes. For PANTHER analysis, we used statistics overrepresentation (i.e., the default setting) to compare classifications of multiple clusters of lists with a reference list to statistically identify the over- or under-representation of PANTHER ontologies. Significance was set to a p value of .05.
Differentially expressed proteins were imported into IPA to identify canonical pathways present exclusively in either naïve or primed secretomes. Fischer's exact test was used to calculate a p value that would determine the probability that the association between genes in the dataset and canonical pathway could be explained by chance alone. Significance was set to .05. The Overlapping Canonical Pathway analysis feature in IPA allows identification of clusters of pathways related by shared protein in user's analysis results.
For each potential upstream regulator (UR) an overlap p value is computed. The overlap p value calls likely upstream regulators based on significant overlap between data set proteins and known targets regulated by a UR. The purpose of the overlap p value is to identify UR that can explain the observed protein expression changes. The overlap p value measures whether there is a statistically significant overlap between the dataset proteins and the proteins that are regulated by a UR. It is calculated using Fisher's Exact Test, and significance was set to p values < 0.01.
Results
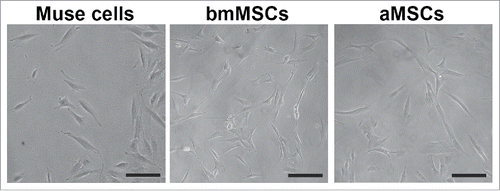
We isolated and cultivated bmMSCs, aMSCs and Muse cells as reported in methods (). We collected conditioned media (secretomes) from early days in vitro cultures (passage 2–3). We performed LC-MS/MS analyses on peptides from the tryptic digestion of secretome samples to identify protein secretome composition. Several hundred proteins were identified in secretomes by using high-resolution MS followed by classification of identified proteins on the Protein Metrics database (Suppl. Files 1).
Figure 1. Cell cultures of Muse cells and MSCs. The figure shows representative fields of cell cultures from which we collected secretomes. Black bar represents 100 microns.
PANTHER gene ontology analysis
Gene Ontology (GO) is an enrichment analysis that allows evaluation of the relative frequency of ontology terms (i.e. biological functions) in the proteomic profile of interest. This consents the identification of ontology terms that occur more frequently in a given data set when compared with a reference protein dataset. GO relies on a database, which groups ontology terms in 3 classes: molecular functions, biological processes, and cellular components.
We used PANTHER gene ontology enrichment analysis to match our experimental data onto reference ontology terms, this allowed ordering systematically our data to get biological information in a hypothesis free manner.
Our GO analysis focused on 2 classes of ontological terms: biological functions and molecular processes. For each of the 3 secretomes we identified several ontologies, as shown in supplementary file 2. An in depth analysis of GO results showed that the great majority of the identified ontological terms were in common among the bmMSC, aMSC and Muse cell secretomes. In detail, the 3 secretomes contained factors that belonged to the following classes: extracellular matrix/cytoskeleton; metabolic processes; ox-redox factors; and components of immune system process ().
Table 1. Panther GO ontologies found in the secretome of Muse cells, bmMSCs and aMSCs. In the table are reported ontologies belonging to “biological process” and “molecular function” classes, which are in common among the 3 cell types we analyzed.
IPA analysis to identify canonical pathways
The ontology terms we found in our experimental data sets with GO analysis, while gave a global portrayal of biological functions that can be associated with secretomes, did not allow identification of the most important proteins in the analyzed proteomes. For this reason, we use IPA analysis to obtain a deeper insight on the most meaningful secretome proteins.
We performed an IPA canonical pathway analysis to attribute proteins present in secretomes to classically characterized signaling paths (canonical pathways), given the assumption that the connectivity of a protein found in a secretome reflects its importance for the secretome's functions.
The proteins identified in the secretomes of bmMSCs, aMSCs and Muse cells could be attributed to hundreds of canonical pathways, as shown in supplementary file 3. We performed a Venn diagram to combine data of all experimental conditions to find the common and specific canonical pathways among the 3 different secretomes (Suppl. File 4). We identified 159 canonical pathways present in 3 secretomes, while 88 were exclusively found in Muse cell secretomes and 49 were shared by bmMSC and aMSC secretomes. We did not evidence canonical pathways exclusively attributed to bmMSCs or aMSCs. This result indicates that the 3 cell populations share a common signaling network, while Muse cells have specific pathways not present in the other populations.
We then focused our attention on the canonical pathways that were represented by 3 or more identified proteins, in order to refine our research and curtail the data to analyze. This also considering that the more a pathway is represented the more may have a significant biological function. Only the 159 common canonical pathways felt in this condition and on these we centered our further investigations.
Canonical pathways that are reported in supplementary file 4 were identified with IPA analysis by evaluating if the likelihood that the association between a set of proteins present in our experimental secretome datesets and a given process or pathway was due to random chance. The smaller the p-value the less likely that the association was random and the more significant the association. This statistical approach alone may not support the determination of pathways that have a true biological meaning. Determination of which canonical pathways belong to overlapping networks reduces the risk of identifying canonical pathways in the experimental dataset that have statistical and not functional significance. Overlapping networks were assembled considering that a given protein may be a part of different pathways, based on the assumption that the more a protein was present in overlapping pathways, the more influence it had on the secretome's activities.
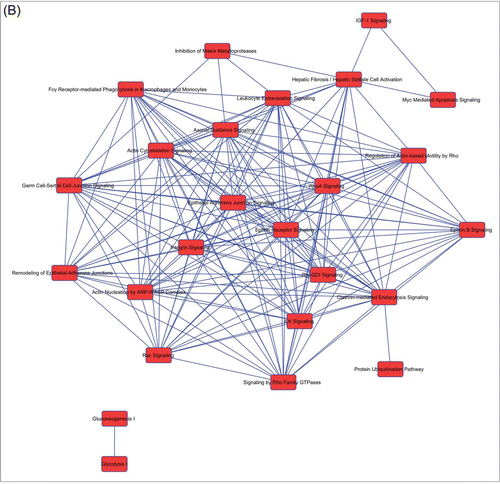
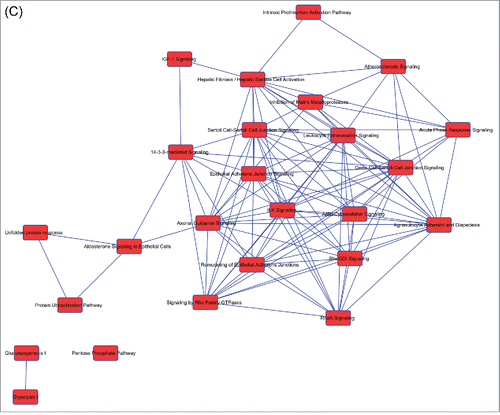
In the 3 secretome data sets only part of 159 canonical pathways were in overlapping networks (). In detail, there were overlapping networks present in the 3 secretomes and others exclusively identified in Muse cell secretomes ().
Figure 2. Overlapping networks. 159 canonical pathways were in common among Muse cells, bmMSCs and aMSCs. We determined for each cell type which of these belonged to overlapping networks. The picture shows overlapping networks in Muse cells (A), in bmMSCs (B) and aMSCs (C).
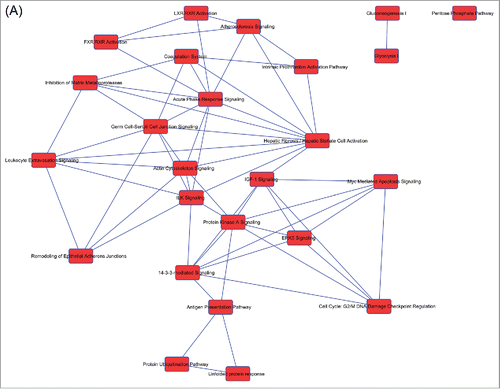
Table 2. List of canonical pathways present in overlapping networks. Some canonical pathways were in the overlapping networks of the 3 cell types, while others were exclusively present in Muse cells. For each cell type the presence of a canonical pathway in overlapping networks is indicated with X.
The overlapping canonical pathways that we found in all secretomes belonged to some of the ontologies we identified with GO: extracellular matrix remodeling, metabolic processes and immunodulation. In addition, the secretomes of bmMSCs and aMSCs shared overlapping pathways that belonged to Rho Signaling, while 7 overlapping canonical pathways were specifically present in Muse cells ().
Proteins expressed exclusively in secretome of Muse cells
Venn analysis of proteins identified with LC-MS/MS analysis on secretome samples showed that 56 proteins were exclusively present in the secretome of Muse cells, while 145 and 51 were only in bmMSC and aMSC secretomes, respectively (Suppl. File 5). Of interest, most of these proteins belonged to the classes we identified with the GO analysis.
Upstream regulators of secretome protein production and/or activity
The IPA Upstream Regulator analysis allows the identification of the cascade of upstream regulatory molecules that can explain the observed gene expression changes enabling to generate likely mechanistic hypotheses.Citation22 Upstream regulators are transcription factors and any other small molecule that has been observed experimentally to affect the expression of genes in the datasets.
We carried out IPA upstream investigation followed by Venn diagram analysis to determine upstream factors exclusively present in a specific cell population as well as factors in common among MSCs and Muse cells (, Suppl. Files 6, 7). We focused our attention on upstream cytokines and growth factors since they play a key role in regulation of stem cell functions and also in immunomodulatory properties of MSCs.Citation7,17 Moreover, we looked also at transcription factors and miRNAs as general regulators of gene expression. The production of Muse cell secretome may rely on the exclusive activity of 7 cytokines, 3 growth factors, 67 transcription factors and 4 miRNAs (Suppl. File 7).
Table 3. Common and Muse cells specific upstream regulators.
Discussion
MSCs release many biologically active factors that have profound effects on local cellular dynamics. A thorough examination of MSC secretome seems essential for understanding the physiological functions exerted by these cells in our organism and also for rational cellular therapy design and for the improvement of existing therapies.
In this setting, studies on secretome of Muse cells, which appear to represent a subset of MSCs with pluripotent features, may shed light on pathways that are associated with specific functions of Muse cells.
Several findings aimed to analyze only specific components of secretomes, such as the released factors affecting extracellular matrix remodeling, proteins involved in inflammation (interleukins, chemokines), as well as those that affect cell proliferation, differentiation and survival (growth factors). We aimed to perform an unbiased analysis of secretomes and for this reason we carried a LC-MS/MS proteome identification and subsequent gene ontology evaluation of proteins released by MSCs and Muse cells.
Common networks among bmMSCs, aMSCs and Muse cells
GO analysis allowed the identification of protein classes (ontologies) that occurred more frequently in bmMSCs, aMSCs and Muse cells. Indeed, the 3 cell populations secreted factors that belong to the same classes: extracellular matrix/cytoskeleton; metabolic processes; ox-redox factors; and components of inflammation phenomenon (, Suppl. File 2). This common background suggests that the identified biological processes and molecular functions are part of the core activities exerted by MSCs and Muse cells. It is well known that MSCs secrete factors involved in regulation of ox-redox status and inflammatory process, here, for the first time, we evidenced that these features are also present in a specific MSC subpopulation, i.e., Muse cells.
IPA analysis by looking at protein-protein interactions allowed the selection of the most meaningful proteins belonging to classes identified in GO studies.
For bmMSCs, aMSCs and Muse cells the proteins present in the secretome could be attributed to hundreds of canonical pathways (Suppl. File 3). This scrutiny was followed by Venn diagram analysis to identify canonical pathways present exclusively in a specific cell population as well as pathways in common among MSCs and Muse cells (Suppl. File 4).
The subsequent identification of overlapping canonical pathways abridged the number of pathways for every experimental condition permitting to determine the most biologically relevant canonical pathways ().
The overlapping canonical pathways that were in common among the analyzed cell populations further underlined the importance of the ontology classes found by Panther GO. In detail, extracellular matrix remodeling may occur through the following pathways i) Remodeling of Epithelial Adherens Junctions; ii) Inhibition of Matrix Metalloproteases; iii) Actin Cytoskeleton Signaling. The metabolic processes appear related mainly to Glycolysis-, Gluconeogenesis- and IGF-1-related pathways. Of interest, immunodulation ontology class appears connected to Leucocyte Extravasation Signaling. In agreement with this, a recent finding showed that MSC immunomodulatory properties may rely not only their anti-proliferative action on lymphocytes but also on the impairment of leukocyte migratory potential.Citation23
Networks exclusively present in MSCs or Muse cells
The bmMSCs and aMSCs share some overlapping pathways that belong to Rho Signaling. These pathways are involved in several cellular functions and it is difficult to identify a specific activity that may be related to MSC secreted proteins that are part of Rho canonical pathways. Some findings claim a role for Rho signaling in MSC differentiation and migration processes but further investigations are needed to validate this hypothesis.Citation24-27
Seven overlapping canonical pathways were specifically present in Muse cells (, ). A closer look to these signaling networks permit to identify the biological activities that they may regulate: apoptosis; stem cell self-renewal; immunomodulation.
Muse cells secrete factors involved in regulation of cell cycle and apoptosis
The proteins belonging to i) Cell Cycle: G2/M DNA Damage Checkpoint Regulation and ii) ERK5 Signaling pathways may play a key role in cell survival and anti-apoptotic activities. This hypothesis arose from analysis of proteins present in Muse secretome that belong to these pathways (, Suppl. File 3).
The 14-3-3 proteins (also named YWHAQ, YWHAG, YWHAE, YWHAZ, YWHAB) are a family of highly conserved acidic 30 kDa molecules that form stable homo- and heterodimers. They bind to their phospho-Serine and phospho-Threonine-containing ligands to regulate a wide range of cellular phenomena. Several studies evidenced that 14-3-3 proteins play a key role in regulation of cell cycle and of cell response to DNA damage following internal or external injury. These proteins may act as chaperonin-like to reduce cellular stress and subsequent apoptosis. Some 14-3-3 isoforms have the capacity to inactivate the pro-apoptotic protein BAD by preventing its negative effect on the pro-survival protein Bcl-XL.Citation28,29 The fact that Muse cell secretome contains most of the 14-3-3 isoforms involved in anti-apoptotic activity strongly suggests that stress enduring capacity of Muse cells may be associated also to the secretion of pro-survival factors that may act in autocrine/paracrine manner.
Muse cells secrete factors that may play a role in stem cell self-renewal
The proteins belonging to i) Protein Kinase A Signaling; ii) FXR/RXR Activation and iii) LXR/RXR Activation pathways may play a role in regulation of stemness of Muse cells ().
PKA pathway is involved in multiple aspects of cell biology. These pleiotropic effects arise from the central position this pathway has among the hundreds of other pathways governing the cell's functions.Citation30 We analyzed the proteins present in Muse secretome that belong to PKA network to find the preeminent role that this pathway may play in Muse autocrine/paracrine signaling.
AKAP13 protein anchors cAMP-dependent protein kinase (PKA) and acts as an adapter protein to selectively couple G α-13 and Rho. Ohgushi and colleagues found that this signaling sustains proliferation, growth and pluripotency of human embryonic stem cells.Citation31
GNAS protein (Guanine nucleotide-binding proteins G protein) function as transducers in numerous signaling pathways including that involved in the activation of adenylyl cyclases. Iglesias-Bartolome and colleagues evidenced that GNAS-PKA pathway has an important role in promoting proper homeostasis of epithelial stem cells in hair follicle by limiting excessive proliferation that may lead to stem cell exhaustion.Citation32
In agreement with these findings there is a report showing that PKA pathway exert a role in self-renewal and differentiation of mouse embryonic stem cells.Citation33
The retinoid X receptors (RXRs) are involved in many cellular processes ranging from cellular proliferation to lipid metabolism. This depends also on the capacity to dimerize with different nuclear receptors. For example, RXRs may dimerize with the liver X receptor (LXR) or with the farnesoid X receptor (FXR). LXR/RXR and FXR/RXR signaling networks play a major role in liver and intestine physiology, respectively.Citation34 Nevertheless, recent findings evidence a role of these pathways, or at least of some related proteins, in promoting cell regeneration both in normal and cancer tissues.Citation35-37 In addition, SERPINF1 (also known as PEDF) that is secreted by Muse cells and belong both to LXR/RXR and FXR/RXR pathways promotes self-renewal a maintaining multipotency of several stem cell types.Citation38-40
Muse cells secrete factors that may regulate immune system functions
The proteins belonging to i) Antigen presentation pathway; ii) Coagulation system; iii) FXR/RXR Activation and iiii) LXR/RXR Activation pathways may play a role in immunomodulation ().
There are several serpins in the coagulation system network present in Muse cell secretome. Serpins are serine proteases whit a fundamental role in the immune system. They are involved in several processes, such as migration, phagocytosis and elimination of virally infected and cancerous cells.Citation41,42 Alpha 2 macroglobulin (A2M) is another protein belonging to coagulation system network (). A2M is the major endoprotease inhibitor in blood and there are findings showing that it has a potent immunosuppressive activity.Citation43-45 Another member of A2M family is the pregnancy zone protein (PZP) that we found express exclusively in Muse cells and not in bmMSCs or aMSCs (Suppl. File 5). This protein is fundamental for a successful pregnancy, since it avoids that maternal immune system attacks the allogeneic fetus.Citation45,46 The presence of A2M and PZP in the secretome of Muse cells may indicate that these cells may have significant immunomodulator capacity.
This hypothesis is further strengthened by identification of other factors in Muse cell secretome that are involved in immune system functions. Looking at proteins of Muse cell secretome we evidenced some components of complement system (i.e. C3, C4A/B) that are part of FXR/RXR and LXR/RXR signaling pathways (). This suggests that these networks may be involved in regulation of immune system properties, besides their possible role in maintaining stemness. Indeed, the complement system is a part of the immune system and consists of a number of small proteins found in the blood that contribute to opsonization, chemotaxis, cell lysis and agglutination.Citation47
Upstream regulatory factors appears related to biological activities associated with secretome content
Given a gene-expression data set, IPA upstream network analysis allows inferring the identity of upstream regulatory molecules. This to have biological insight to the proteins present in the analyzed secretomes.Citation22 Following IPA upstream investigation we performed Venn diagram analysis to identify upstream factors exclusively present in a specific cell population as well as factors in common among MSCs and Muse cells (, Suppl. Files 6, 7).
Several interleukins (IL1A, IL1B, IL3, IL4, IL8, IL13, IL17, IFNγ, TNFα) that are well know regulators of native and adaptive immunity appear as putative upstream regulators of both MSCs and Muse cell secretomes.Citation47 This is in good agreement with immunomodulatory duty exerted by these cells.
Some upstream regulatory cytokines (CRH, LIF) found only in Muse cells suggests a role in control of stem cell identity, self-renewal and protection from pro-apoptotic stress.Citation48-50 This further confirms that Muse secretome may have components that acting in autocrine/paracrine manner could promote cell survival and preserve stemness.
Many upstream growth factors that were identified as common regulators of MSC and Muse cell secretomes have a role in regulating stem cell niche, tissue homeostasis and cell survival. Among these is note to mention VEGF, EGF and FGF that are well know regulators of MSC biology.Citation51-53 The BMP4 and FGF18 growth factors were identified as Muse cell specific upstream regulators. They are involved in heightening stem differentiation potential and control cell commitment.Citation54-56
A huge number of transcription factors were identified with IPA and Venn analysis as potential common upstream regulators of protein products evidenced in MSC and Muse cell secretomes. Their activities cover all the aspect of cell biology and it is difficult to relate a single factor with a specific biological activity that could be ascribed to proteins present in secretomes. Nevertheless, some of them (TP53, JUN, FOS, E2F1, STAT3, SMAD3, KLF2) may be more strictly associated with biological and molecular functions attributed to secretomes of MSCs and Muse cells, such as remodeling of extracellular matrix, metabolic processes, ox-redox processes and regulation of inflammation phenomena ().
IPA and Venn analysis identified also dozens of transcription factors that appear to regulate exclusively the production of Muse cell secretome (). Of note, several factors, such as POU2F1, DLX4, BCL11A, KLF5, GATA3, AIRE and FLH2 are involved in governing stem cell functions (self-renewal, cell fate specification, cell survival under stress).Citation57-63
Only a few miRNAs were found to be putative upstream regulators of proteins present in MSC and Muse cell secretomes (). In detail, mir-122 and mir-133 appear related to functions ascribed to secretomes of MSCs and Muse cells, such as remodeling of extracellular matrix, metabolic processes and stemness control.Citation64-66
Globally speaking, the upstream regulators identified by IPA analysis lend further credit to the putative identified functions of MSC and Muse cells secretomes. Indeed, literature data evidenced that the pinpointed factors do act in controlling cellular process identified by GO and canonical pathway analyses.
Conclusion
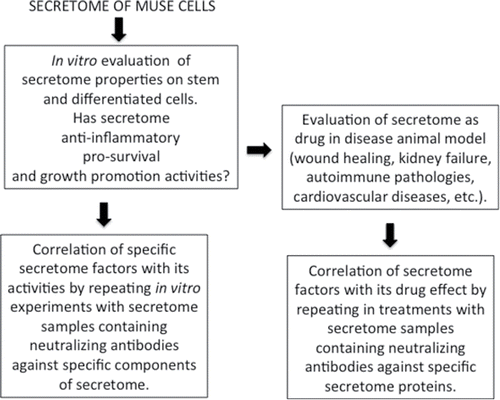
Our studies evidenced that secretomes of MSCs and Muse cells contain factors that regulate extracellular matrix remodeling, ox-redox activities and immune system functions. In addition, Muse cells appear to secrete several factors that may preserve their stem cell features, allow survival under stress conditions and may contribute to their immunomodulation capacity. Further investigations are needed to evaluate the role of the pathways we identified with our study. The importance of these further findings resides on the consideration that paracrine signaling may play a key role in therapeutic performance of Muse cells. We suggest that supplementary studies should be performed according the experimental plan reported in in order to dissect the specific function of identified secreted factors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary Files
Download Zip (1.4 MB)References
- Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest 2004; 113:1701-10; PMID:15199405; http://dx.doi.org/10.1172/JCI200420935
- Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol 2006; 44:742-8; PMID:16469408; http://dx.doi.org/10.1016/j.jhep.2005.10.026
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143-7; PMID:10102814; http://dx.doi.org/10.1126/science.284.5411.143
- Shimizu S, Kitada M, Ishikawa H, Itokazu Y, Wakao S, Dezawa M. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem Biophys Res Commun 2007; 359:915-20; PMID:17573041; http://dx.doi.org/10.1016/j.bbrc.2007.05.212
- Tamai K, Yamazaki T, Chino T, Ishii M, Otsuru S, Kikuchi Y, Iinuma S, Saga K, Nimura K, Shimbo T, et al. PDGFRalpha-positive cells in bone marrow are mobilized by high mobility group box 1 (HMGB1) to regenerate injured epithelia. Proc Natl Acad Sci U S A 2011; 108:6609-14; PMID:21464317; http://dx.doi.org/10.1073/pnas.1016753108
- Terai S, Sakaida I, Yamamoto N, Omori K, Watanabe T, Ohata S, Katada T, Miyamoto K, Shinoda K, Nishina H, et al. An in vivo model for monitoring trans-differentiation of bone marrow cells into functional hepatocytes. J Biochem 2003; 134:551-8; PMID:14607982; http://dx.doi.org/10.1093/jb/mvg173
- Galderisi U, Giordano A. The gap between the physiological and therapeutic roles of mesenchymal stem cells. Med Res Rev 2014; 34:1100-26; PMID:24866817; http://dx.doi.org/10.1002/med.21322
- Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A, et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci U S A 2010; 107:8639-43; PMID:20421459; http://dx.doi.org/10.1073/pnas.0911647107
- Dezawa M. Muse cells provide the pluripotency of mesenchymal stem cells: direct contribution of Muse cells to tissue regeneration. Cell Transplant 2016; 25:849-61; PMID:26884346
- Tsuchiyama K, Wakao S, Kuroda Y, Ogura F, Nojima M, Sawaya N, Yamasaki K, Aiba S, Dezawa M. Functional melanocytes are readily reprogrammable from multilineage-differentiating stress-enduring (muse) cells, distinct stem cells in human fibroblasts. J Invest Dermatol 2013; 133:2425-35; PMID:23563197; http://dx.doi.org/10.1038/jid.2013.172
- Kinoshita K, Kuno S, Ishimine H, Aoi N, Mineda K, Kato H, Doi K, Kanayama K, Feng J, Mashiko T, et al. Therapeutic potential of adipose-derived SSEA-3-positive muse cells for treating diabetic skin ulcers. Stem Cell Transl Med 2015; 4:146-55; PMID:25561682; http://dx.doi.org/10.5966/sctm.2014-0181
- Katagiri H, Kushida Y, Nojima M, Kuroda Y, Wakao S, Ishida K, Endo F, Kume K, Takahara T, Nitta H, et al. A Distinct subpopulation of bone marrow mesenchymal stem cells, muse cells, directly commit to the replacement of liver components. Am J Transplant 2016; 16:468-83; PMID:26663569; http://dx.doi.org/10.1111/ajt.13537
- Uchida H, Morita T, Niizuma K, Kushida Y, Kuroda Y, Wakao S, Sakata H, Matsuzaka Y, Mushiake H, Tominaga T, et al. Transplantation of unique subpopulation of fibroblasts, muse cells, ameliorates experimental stroke possibly via robust neuronal differentiation. Stem Cells 2016; 34:160-73; PMID:26388204; http://dx.doi.org/10.1002/stem.2206
- Gallina C, Turinetto V, Giachino C. A new paradigm in cardiac regeneration: The mesenchymal stem cell secretome. Stem Cells Int 2015; 2015:765846; PMID:26074978; http://dx.doi.org/10.1155/2015/765846
- Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell stem cell 2012; 10:244-58; PMID:22385653; http://dx.doi.org/10.1016/j.stem.2012.02.005
- Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U, Chambery A. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis 2013; 4:e911; PMID:24201810; http://dx.doi.org/10.1038/cddis.2013.445
- Zimmerlin L, Park TS, Zambidis ET, Donnenberg VS, Donnenberg AD. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie 2013; 95:2235-45; PMID:23747841; http://dx.doi.org/10.1016/j.biochi.2013.05.010
- Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 2013; 95:2196-211; PMID:23880644; http://dx.doi.org/10.1016/j.biochi.2013.07.015
- Ozcan S, Alessio N, Acar MB, Toprak G, Gonen ZB, Peluso G, Galderisi U. Myeloma cells can corrupt senescent mesenchymal stromal cells and impair their anti-tumor activity. Oncotarget 2015; 6:39482-92; PMID:26498687
- Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 2012; 21:2724-52; PMID:22468918; http://dx.doi.org/10.1089/scd.2011.0722
- Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 2006; 1:2856-60; PMID:17406544; http://dx.doi.org/10.1038/nprot.2006.468
- Kramer A, Green J, Pollard J, Jr., Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014; 30:523-30; PMID:24336805; http://dx.doi.org/10.1093/bioinformatics/btt703
- Benvenuto F, Voci A, Carminati E, Gualandi F, Mancardi G, Uccelli A, Vergani L. Human mesenchymal stem cells target adhesion molecules and receptors involved in T cell extravasation. Stem Cell Res Ther 2015; 6:245; PMID:26651832; http://dx.doi.org/10.1186/s13287-015-0222-y
- Jaganathan BG, Ruester B, Dressel L, Stein S, Grez M, Seifried E, Henschler R. Rho inhibition induces migration of mesenchymal stromal cells. Stem Cells 2007; 25:1966-74; PMID:17510214; http://dx.doi.org/10.1634/stemcells.2007-0167
- Liu X, Zhang Z, Yan X, Liu H, Zhang L, Yao A, Guo C, Liu X, Xu T. The Rho kinase inhibitor Y-27632 facilitates the differentiation of bone marrow mesenchymal stem cells. J Mol Histol 2014; 45:707-14; PMID:25178638; http://dx.doi.org/10.1007/s10735-014-9594-z
- Tai IC, Wang YH, Chen CH, Chuang SC, Chang JK, Ho ML. Simvastatin enhances Rho/actin/cell rigidity pathway contributing to mesenchymal stem cells' osteogenic differentiation. Int J Nanomedicine 2015; 10:5881-94; PMID:26451103
- Xu T, Wu M, Feng J, Lin X, Gu Z. RhoA/Rho kinase signaling regulates transforming growth factor-beta1-induced chondrogenesis and actin organization of synovium-derived mesenchymal stem cells through interaction with the Smad pathway. Int J Mol Med 2012; 30:1119-25; PMID:22922645
- Clapp C, Portt L, Khoury C, Sheibani S, Norman G, Ebner P, Eid R, Vali H, Mandato CA, Madeo F, et al. 14-3-3 protects against stress-induced apoptosis. Cell Death Dis 2012; 3:e348; PMID:22785534; http://dx.doi.org/10.1038/cddis.2012.90
- Gardino AK, Yaffe MB. 14-3-3 proteins as signaling integration points for cell cycle control and apoptosis. Semin Cell Dev Biol 2011; 22:688-95; PMID:21945648; http://dx.doi.org/10.1016/j.semcdb.2011.09.008
- Cooper JM, Hausman RE. The Cell: A Molecular Approach. Sunderland, MA, USA: Sinauer Associates, Inc, 2016.
- Ohgushi M, Minaguchi M, Sasai Y. Rho-Signaling-Directed YAP/TAZ Activity Underlies the Long-Term Survival and Expansion of Human Embryonic Stem Cells. Cell Stem Cell 2015; 17:448-61; PMID:26321201; http://dx.doi.org/10.1016/j.stem.2015.07.009
- Iglesias-Bartolome R, Torres D, Marone R, Feng X, Martin D, Simaan M, Chen M, Weinstein LS, Taylor SS, Molinolo AA, et al. Inactivation of a Galpha(s)-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol 2015; 17:793-803; PMID:25961504; http://dx.doi.org/10.1038/ncb3164
- Faherty S, Fitzgerald A, Keohan M, Quinlan LR. Self-renewal and differentiation of mouse embryonic stem cells as measured by Oct4 expression: the role of the cAMP/PKA pathway. In Vitro Cell Dev Biol Anim 2007; 43:37-47; PMID:17570033; http://dx.doi.org/10.1007/s11626-006-9001-5
- Lefebvre P, Benomar Y, Staels B. Retinoid X receptors: common heterodimerization partners with distinct functions. Trends Endocrinol Metab 2010; 21:676-83; PMID:20674387; http://dx.doi.org/10.1016/j.tem.2010.06.009
- Meng Q, Chen X, Wang C, Liu Q, Sun H, Sun P, Peng J, Liu K. Alisol B 23-acetate promotes liver regeneration in mice after partial hepatectomy via activating farnesoid X receptor. Biochem Pharmacol 2014; 92:289-98; PMID:25278094; http://dx.doi.org/10.1016/j.bcp.2014.09.009
- Ng KY, Chai S, Tong M, Guan XY, Lin CH, Ching YP, et al. C-terminal truncated hepatitis B virus X protein promotes hepatocellular carcinogenesis through induction of cancer and stem cell-like properties. Oncotarget 2016; 7:24005-17.
- Sacchetti P, Sousa KM, Hall AC, Liste I, Steffensen KR, Theofilopoulos S, Parish CL, Hazenberg C, Richter LA, Hovatta O, et al. Liver X receptors and oxysterols promote ventral midbrain neurogenesis in vivo and in human embryonic stem cells. Cell Stem Cell 2009; 5:409-19; PMID:19796621; http://dx.doi.org/10.1016/j.stem.2009.08.019
- Elahy M, Baindur-Hudson S, Dass CR. The emerging role of PEDF in stem cell biology. J Biomed Biotechnol 2012; 2012:239091; PMID:22675247; http://dx.doi.org/10.1155/2012/239091
- Ho TC, Chen SL, Wu JY, Ho MY, Chen LJ, Hsieh JW, Cheng HC, Tsao YP. PEDF promotes self-renewal of limbal stem cell and accelerates corneal epithelial wound healing. Stem Cells 2013; 31:1775-84; PMID:23553951; http://dx.doi.org/10.1002/stem.1393
- Zille M, Riabinska A, Terzi MY, Balkaya M, Prinz V, Schmerl B, Nieminen-Kelhä M, Endres M, Vajkoczy P, Pina AL. Influence of pigment epithelium-derived factor on outcome after striatal cerebral ischemia in the mouse. PLoS One 2014; 9:e114595; PMID:25470280; http://dx.doi.org/10.1371/journal.pone.0114595
- Gatto M, Iaccarino L, Ghirardello A, Bassi N, Pontisso P, Punzi L, Shoenfeld Y, Doria A. Serpins, immunity and autoimmunity: old molecules, new functions. Clin Rev Allergy Immunol 2013; 45:267-80; PMID:23325331; http://dx.doi.org/10.1007/s12016-013-8353-3
- Mangan MS, Kaiserman D, Bird PI. The role of serpins in vertebrate immunity. Tissue Antigens 2008; 72:1-10; PMID:18498291; http://dx.doi.org/10.1111/j.1399-0039.2008.01059.x
- Francis DM, Shenton BK, Proud G, Taylor RM. Immunosuppressive plasma factors in malignant disease. Aust N Z J Surg 1985; 55:111-20; PMID:2412540; http://dx.doi.org/10.1111/j.1445-2197.1985.tb00869.x
- koo PH, Khol M. {alpha}2-Macroglobulin (a2M) Mediates T Cell Suppression via a Peptide Sequence in its Cytokine-Binding Site. Faseb Journal 2008; 22:1(Suppl. 848.25)
- Tayade C, Esadeg S, Fang Y, Croy BA. Functions of alpha 2 macroglobulins in pregnancy. Mol Cell Endocrinol 2005; 245:60-6; PMID:16297527; http://dx.doi.org/10.1016/j.mce.2005.10.004
- Skornicka EL, Kiyatkina N, Weber MC, Tykocinski ML, Koo PH. Pregnancy zone protein is a carrier and modulator of placental protein-14 in T-cell growth and cytokine production. Cell Immunol 2004; 232:144-56; PMID:15882859; http://dx.doi.org/10.1016/j.cellimm.2005.03.007
- Murphy K, Travers P, Walport M. Janeway's Immunobiology. New York: Garland Science, 2007.
- Koutmani Y, Politis PK, Elkouris M, Agrogiannis G, Kemerli M, Patsouris E, Remboutsika E, Karalis KP. Corticotropin-releasing hormone exerts direct effects on neuronal progenitor cells: implications for neuroprotection. Mol Psychiatry 2013; 18:300-7; PMID:23380766; http://dx.doi.org/10.1038/mp.2012.198
- Kovacs KJ. CRH: the link between hormonal-, metabolic- and behavioral responses to stress. J Chem Neuroanat 2013; 54:25-33; PMID:23774011; http://dx.doi.org/10.1016/j.jchemneu.2013.05.003
- Onishi K, Zandstra PW. LIF signaling in stem cells and development. Development 2015; 142:2230-6; PMID:26130754; http://dx.doi.org/10.1242/dev.117598
- Berendsen AD, Olsen BR. How vascular endothelial growth factor-A (VEGF) regulates differentiation of mesenchymal stem cells. J Histochem Cytochem 2014; 62:103-8; PMID:24309509; http://dx.doi.org/10.1369/0022155413516347
- Coutu DL, Francois M, Galipeau J. Inhibition of cellular senescence by developmentally regulated FGF receptors in mesenchymal stem cells. Blood 2011; 117:6801-12; PMID:21527526; http://dx.doi.org/10.1182/blood-2010-12-321539
- Salehinejad P, Alitheen NB, Mandegary A, Nematollahi-Mahani SN, Janzamin E. Effect of EGF and FGF on the expansion properties of human umbilical cord mesenchymal cells. In Vitro Cell Dev Biol Anim 2013; 49:515-23; PMID:23708920; http://dx.doi.org/10.1007/s11626-013-9631-3
- Jeon E, Yun YR, Kang W, Lee S, Koh YH, Kim HW, Suh CK, Jang JH. Investigating the role of FGF18 in the cultivation and osteogenic differentiation of mesenchymal stem cells. PLoS One 2012; 7:e43982; PMID:22937141; http://dx.doi.org/10.1371/journal.pone.0043982
- Taha MF, Javeri A, Majidizadeh T, Valojerdi MR. Both BMP4 and serum have significant roles in differentiation of embryonic stem cells to primitive and definitive endoderm. Cytotechnology 2016; 68:1315-24; PMID:26008149
- Yang Y, Adachi K, Sheridan MA, Alexenko AP, Schust DJ, Schulz LC, Ezashi T, Roberts RM. Heightened potency of human pluripotent stem cell lines created by transient BMP4 exposure. Proc Natl Acad Sci U S A 2015; 112:E2337-46; PMID:25870291; http://dx.doi.org/10.1073/pnas.1504778112
- Bin G, Jiarong Z, Shihao W, Xiuli S, Cheng X, Liangbiao C, Ming Z. Aire promotes the self-renewal of embryonic stem cells through Lin28. Stem Cells Dev 2012; 21:2878-90; PMID:22540148; http://dx.doi.org/10.1089/scd.2012.0097
- Frelin C, Herrington R, Janmohamed S, Barbara M, Tran G, Paige CJ, Benveniste P, Zuñiga-Pflücker JC, Souabni A, Busslinger M, et al. GATA-3 regulates the self-renewal of long-term hematopoietic stem cells. Nat Immunol 2013; 14:1037-44; PMID:23974957; http://dx.doi.org/10.1038/ni.2692
- Hammoud AA, Kirstein N, Mournetas V, Darracq A, Broc S, Blanchard C, Zeineddine D, Mortada M, Boeuf H. Murine embryonic stem cell plasticity is regulated through Klf5 and maintained by metalloproteinase MMP1 and hypoxia. PLoS One 2016; 11:e0146281; PMID:26731538
- Infante A, Gago A, de Eguino GR, Calvo-Fernandez T, Gomez-Vallejo V, Llop J, Schlangen K, Fullaondo A, Aransay AM, Martín A, et al. Prelamin A accumulation and stress conditions induce impaired Oct-1 activity and autophagy in prematurely aged human mesenchymal stem cell. Aging (Albany NY) 2014; 6:264-80; PMID:24753226; http://dx.doi.org/10.18632/aging.100651
- Jo A, Denduluri S, Zhang B, Wang Z, Yin L, Yan Z, Kang R, Shi LL, Mok J, Lee MJ, et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis 2014; 1:149-61; PMID:25685828; http://dx.doi.org/10.1016/j.gendis.2014.09.004
- Powers AN, Satija R. Single-cell analysis reveals key roles for Bcl11a in regulating stem cell fate decisions. Genome Biol 2015; 16:199; PMID:26390863; http://dx.doi.org/10.1186/s13059-015-0778-y
- Tamaoki N, Takahashi K, Aoki H, Iida K, Kawaguchi T, Hatakeyama D, Inden M, Chosa N, Ishisaki A, Kunisada T, et al. The homeobox gene DLX4 promotes generation of human induced pluripotent stem cells. Sci Rep 2014; 4:7283; PMID:25471527; http://dx.doi.org/10.1038/srep07283
- Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, et al. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res 2009; 104:170-8, 6p following 8; PMID:19096030; http://dx.doi.org/10.1161/CIRCRESAHA.108.182535
- Li J, Ghazwani M, Zhang Y, Lu J, Li J, Fan J, Gandhi CR, Li S. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J Hepatol 2013; 58:522-8; PMID:23178710; http://dx.doi.org/10.1016/j.jhep.2012.11.011
- Song K, Kwon H, Han C, Zhang J, Dash S, Lim K, Wu T. Active glycolytic metabolism in CD133(+) hepatocellular cancer stem cells: regulation by MIR-122. Oncotarget 2015; 6:40822-35; PMID:26506419