Proliferating cell nuclear antigen (PCNA) is a critical regulator of DNA replication in the S phase of the cell cycle. It forms a homotrimer DNA clamp that enhances the processivity of DNA polymerases.Citation1 At the beginning of DNA replication, PCNA is loaded onto DNA by the Replication Factor C (RFC) complex, and facilitates continuous synthesis of DNA by DNA polymerases.Citation2 Upon completion of DNA synthesis, PCNA is unloaded from DNA by the RFC-like Elg1 complex.Citation3 Although this major PCNA-unloader has been identified, regulation of PCNA-unloading is not well understood.
The KAT6 acetyltransferases are conserved in a wide range of eukaryotes, and are generally involved in cell cycle regulation.Citation4 The Drosophila KAT6, Enok, was found to play critical roles in neuroblast proliferation,Citation5 but information regarding the underlying mechanisms has been long lacking. In our recently published study, we sought to investigate how Enok regulates cell cycle progression by identifying its novel interacting partners.Citation6 We affinity-purified the Enok complex from S2 cells and, interestingly, the Elg1 complex co-purified with Enok. Co-immunoprecipitation assays using antibodies against Enok or Elg1 verified that endogenous Enok and Elg1 interact with each other. This Enok-Elg1 interaction is mediated by the scaffold subunit in the Enok complex, Br140 (; top left), as in vitro association of recombinant Enok with Elg1 required the presence of Br140.
Figure 1. Model of regulation of mitotic cell cycle and endocycle by Enok. Top: The Enok complex interacts with the Elg1 complex and inhibits its PCNA-unloading function to promote the G1/S progression. Speculated underlying mechanisms include sequestration and acetylation of Elg1 by the Enok complex (left). In the absence of Enok, the hyperactive Elg1 complex unloads PCNA from chromatin promiscuously, resulting in G1/S transition delay (right). Bottom: Normal endoreplication in nurse cells depends on efficient PCNA-unloading by the Elg1 complex (left). When the levels of Elg1 is reduced, inefficient PCNA-unloading caused defects in nurse cell endoreplication (middle). Depletion of Enok in Elg1-depleted nurse cells increases the activity of the remaining Elg1 and partially rescues the defective endoreplication (Right).
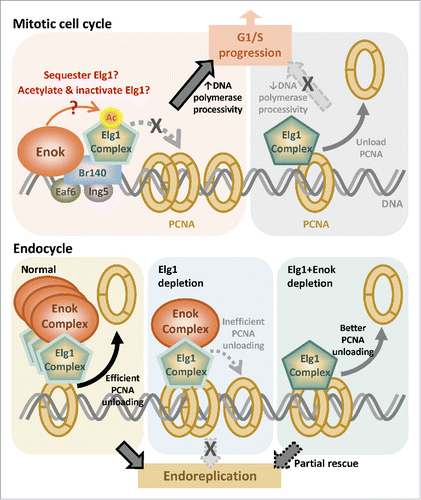
While depletion of Enok in S2 cells did not significantly affect progression through S phase, we found that Enok depletion resulted in a block at the G1/S transition and an increased rate of G2/M progression. The altered G2/M progression rate in Enok-depleted cells is independent of Elg1. However, the G1/S block caused by Enok depletion is partly dependent on Elg1, as reducing Elg1 levels in Enok-depleted cells partially relieved this G1/S block. Since PCNA plays critical roles in DNA replication, the functional interaction between Enok and Elg1 at the G1/S transition suggests a role for Enok in regulating the PCNA-unloading function of Elg1. Indeed, depletion of Enok in S2 cells or Drosophila embryos resulted in reduced levels of PCNA on chromatin without affecting total PCNA levels. Therefore, we proposed that Enok might associate with Elg1 and inhibit its PCNA-unloading function to promote the G1/S transition (; top left). In the absence of Enok, the Elg1 complex becomes hyperactive and removes PCNA from chromatin promiscuously, leading to a G1/S block (; top right).
Our hypothesis that Enok inhibits the Elg1 complex is further supported by the genetic interaction between enok and elg1 in the Drosophila germline (including germline stem cells, cystoblasts, cystocytes, nurse cells and oocytes). Disrupting or knocking down elg1 in the ovary hindered nurse cell endoreplication, causing under-replicated nurse cells and female sterility. Strikingly, knocking down enok partially rescued defective endoreplication in Elg1-depleted nurse cells. This result supports our hypothesis and suggests that reducing Enok levels in Elg1-depleted nurse cells may reduce Enok-mediated inhibition of the remaining Elg1. Thus the PCNA-unloading activity of this residual Elg1 would be higher, and restore moderate levels of endoreplication (; bottom).
Our findings shed light on cell cycle regulation by the KAT6 acetyltransferases. Interestingly, while Enok interacts with the Elg1 complex in Drosophila, the yeast homolog of Enok, Sas3, co-purified with the largest subunit of the RFC complex, Rfc1.Citation7 Thus, this interaction between KAT6 and the RFC/RFC-like complex is conserved between yeast and Drosophila. It is conceivable that the human KAT6, MOZ/MORF, may also contribute to cell cycle regulation by interacting with one of the RFC/RFC-like complexes. Another remaining question is how Enok limits the PCNA-unloading function of Elg1. We have observed that Elg1 remaining on chromatin after high salt extraction was lost upon depletion of Enok. This observation raises the possibility that the Enok complex may sequester the active Elg1 complex away from chromatin-bound PCNA (; top left). In addition, since Enok is a lysine acetyltransferase, it may inhibit the PCNA-interacting ability/ATPase activity of Elg1 by directly acetylating lysine residue(s) in Elg1 (; top left). Taken together, future investigation into the link between KAT6 acetyltransferases and the RFC/RFC-like complexes will advance our understanding of the mechanisms by which DNA replication and cell cycle progression are regulated by this group of proteins.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell 2007; 129:665-79; PMID:17512402; http://dx.doi.org/10.1016/j.cell.2007.05.003
- Majka J, Burgers PMJ. The PCNA–RFC families of DNA clamps and clamp loaders. Prog Nucleic Acid Res Mol Biol 2004; 78:227-60; PMID:15210332http://dx.doi.org/10.1016/S0079-6603(04)78006-X
- Kubota T, Nishimura K, Kanemaki MT, Donaldson AD. The Elg1 replication factor C-like complex functions in PCNA unloading during DNA replication. Mol Cell 2013; 50:273-80; PMID:23499004http://dx.doi.org/10.1016/j.molcel.2013.02.012
- Huang F, Abmayr SM, Workman JL. Regulation of KAT6 acetyltransferases and their roles in cell cycle progression, stem cell maintenance, and human disease. Mol Cell Biol 2016; 36:1900-7; PMID:27185879; http://dx.doi.org/10.1128/MCB.00055-16
- Scott EK, Lee T, Luo L. enok encodes a Drosophila putative histone acetyltransferase required for mushroom body neuroblast proliferation. Current biology: CB 2001; 11:99-104; PMID:11231125; http://dx.doi.org/10.1016/S0960-9822(01)00020-3
- Huang F, Bonni A. A decade of the anaphase-promoting complex in the nervous system. Genes Dev 2016; 30:1198-210; PMID:27198229; http://dx.doi.org/10.1101/gad.274324.115
- Vicente-Muñoz, Romero P, Magraner-Pardo L, Martinez-Jimenez CP, Tordera V, Pamblanco M. Comprehensive analysis of interacting proteins and genome-wide location studies of the Sas3-dependent NuA3 histone acetyltransferase complex. FEBS Open Bio 2014; 4:996-1006; PMID:25473596; http://dx.doi.org/10.1016/j.fob.2014.11.001
