Hyperactivation of the mTOR-AKT pathway frequently contributes to the spread of cancer cells, especially in aggressive breast cancer, to distant organs, promoting the lethal metastatic program. Despite advances in the understanding of this signaling pathway and the development of inhibitors, efforts are still needed to dissect its complexity. This is particularly the case for mTORC2, which constitutes one of the 2 major branches of the mTOR pathway and whose mode of regulation is poorly defined. The mTORC2 complex consists of RICTOR, an evolutionarily conserved protein associated to mTOR, LST8 and SIN1, which is responsible for the phosphorylation of AGC protein kinases (AKT, SGK1 and PKCα). In a recent report,Citation1 we reveal that, in breast cancer cells, the mTORC2 complex, through the RICTOR subunit, can associate with PRICKLE1, a core member of planar cell polarity (PCP) which normally shapes organs during embryogenesis of Metazoans (). PRICKLE1 is a developmental gene upregulated in breast cancer cells which, along with other PCP components, promotes breast cancer cell motility through a WNT-dependent signaling cascade.Citation2,3 We provide evidence that the PRICKLE-RICTOR complex promotes cytoskeleton reorganization of breast cancer cells, cell motility, and proliferation. Cancer cells adopt an invasive phenotype by remodeling their actomyosin cytoskeleton leading to changes in cell shape, and the acquisition of a mesenchymal phenotype. In our study, we observe that cells defective for the PRICKLE1 complex are more spread out, present thicker actin bundles and more stable focal adhesions. We propose that this phenotype is due, at least in part, to a defect of integrin internalization, which is required for focal adhesion dynamics. However what links PRICKLE1 to the regulation of integrin endocytosis remains unknown. Our data further show that the association between PRICKLE1 and RICTOR is positively regulated by MINK1, a prometastatic serine-threonine kinase phosphorylating PRICKLE1.Citation4 As a matter of fact, MINK1 acts as a rheostat not only for the control of the PRICKLE1-RICTOR association, but also for RICTOR - and thus mTORC2 - membrane localization, and induction of AKT phosphorylation (). This PRICKLE1-dependent AKT phosphorylation is rather specific as i) PKCα phosphorylation is insensitive to PRICKLE1 or MINK1 deficiency, and ii) only phosphorylation of AKT at serine 473 (required for signaling) and not at threonine 450 (required for protein stability) is affected.
Figure 1. (Left) localization of Venus-PRICKLE1 at the leading edge of a migratory MDA-MB231 breast cancer cell. (Right) schematic of PRICKLE1 recruitment at the plasma membrane. Phosphorylation of PRICKLE1 by MINK1 leads to the localization of the PRICKLE1-mTORC2 protein complex and to AKT phosphorylation. The four main questions to answer (see text) are numbered in red.
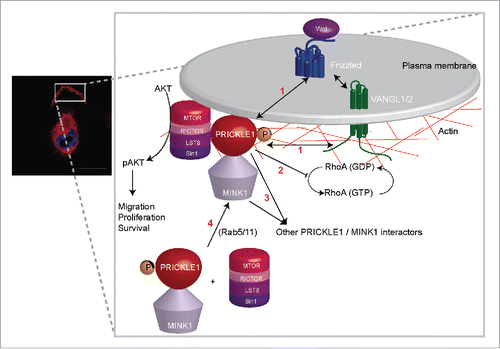
AKT is a pivotal enzyme regulated by phosphorylation, controlling numerous cellular processes such as proliferation, survival and cell motility. Previous work suggested that mTORC2 localization may play an important role in the regulation of AKT phosphorylation.Citation5 Our work suggests that it may be due, in part, to the PRICKLE1 complex which localizes in discrete membrane compartments.Citation1 We obtained some interesting confirmation of our data in clinical samples by showing that, in aggressive triple-negative (also called basal) breast cancers, overexpression of PRICKLE1 correlates with high levels of phosphorylated AKT as well as of 2 AKT substrates (FOXO3A and PRAS40).Citation1
Our findings raise several issues. We will discuss 4 of them (). First, do other PCP components belong to the PRICKLE1-mTORC2-AKT pathway described above? It may well be that VANGL1 and VANGL2, 2 homologous PCP receptor associated with PRICKLE1 and overexpressed in breast cancers,Citation1-3 act upstream with the help of WNT ligands and co-receptors. Upstream regulators may vary with cell context: in drug-resistant melanoma, increased levels of WNT5A and the presence of FRIZZLED7 and RYK PCP receptors correlate with AKT phosphorylation.Citation6 However, hijacking of the mTORC2-AKT pathway by overexpressed PRICKLE1 in cancer cells remains a possibility. Definitive answers should be provided by genetic studies testing the involvement of the mTORC2-AKT pathway in PCP-dependent developmental processes. Second, the role of PRICKLE1 in cell shape regulation requires further investigations. Interestingly, a recent report addressed this issue and showed the importance of Rho signaling.Citation7 However the role of MINK1 in this process remains unknown. Third, as we do not fully recapitulate the phenotypes observed by knock-down of the PRICKLE1-mTORC2 complex with AKT inhibitors, we suspect the involvement of non-AKT signaling, maybe linked to RhoA, associated with PRICKLE1. Some candidate molecules could be certainly selected from the large web of PRICKLE1 interactors recently identified by our lab and others.Citation1,4,7 Fourth, we previously showed that Rab5 and Rab11 are required for the asymmetrical distribution of the PRICKLE1 protein complex at the plasma membraneCitation4. As these small GTPases are implicated in cancer progression, their role in PRICKLE1-dependent cancer cell migration will have to be tested.
Our report reveals a previously unidentified link between the developmental PCP pathway and mTORC2, in breast cancer, adding further support to recent data implicating this pathway in tumorigenesis. Further, it paves the way to the development of strategies for potential inhibition of this hyperactive signaling cascade, either directly (inhibition of MINK1 or PRICKLE1-RICTOR interaction) or indirectly (AKT inhibition) in the future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Daulat AM, Bertucci F, Audebert S, Sergé A, Finetti P, Josselin E, Castellano R, Birnbaum D, Angers S, Borg J-P. PRICKLE1 contributes to cancer cell dissemination through its interaction with mTORC2. Dev Cell 2016; 37:311-25; PMID:27184734; http://dx.doi.org/10.1016/j.devcel.2016.04.011
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012; 151:1542-56; PMID:23260141; http://dx.doi.org/10.1016/j.cell.2012.11.024
- Puvirajesinghe TM, Bertucci F, Jain A, Scerbo P, Belotti E, Audebert S, Sebbagh M, Lopez M, Brech A, Finetti P, et al. Identification of p62/SQSTM1 as a component of non-canonical Wnt VANGL2–JNK signalling in breast cancer. Nat Commun 2016; 7:10318; PMID:26754771; http://dx.doi.org/10.1038/ncomms10318
- Daulat AM, Luu O, Sing A, Zhang L, Wrana JL, McNeill H, Winklbauer R, Angers S. Mink1 regulates β-catenin-independent Wnt signaling via prickle phosphorylation. Mol Cell Biol 2012; 32:173-85; PMID:22037766; http://dx.doi.org/10.1128/MCB.06320-11
- Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J 2008; 27:1932-43; PMID:18566586; http://dx.doi.org/10.1038/emboj.2008.120
- Anastas JN, Kulikauskas RM, Tamir T, Rizos H, Long GV, von Euw EM, Yang P-T, Chen H-W, Haydu L, Toroni RA, et al. WNT5A enhances resistance of melanoma cells to targeted BRAF inhibitors. J Clin Invest 2014; 124: 2877-90; PMID:24865425; http://dx.doi.org/10.1172/JCI70156
- Zhang L, Luga V, Armitage SK, Musiol M, Won A, Yip CM, Plotnikov SV, Wrana JL. A lateral signalling pathway coordinates shape volatility during cell migration. Nat Commun 2016; 7:11714; PMID:27226243; http://dx.doi.org/10.1038/ncomms11714
