Chromosome segregation during mitosis depends on the assembly of a bipolar microtubule-based machine, the mitotic spindle. Different signaling pathways regulating microtubule nucleation, dynamics and spatial organization contribute to this process. These pathways control different Spindle Assembly Factors (SAFs) and operate on or around centrosomes, chromosomes (chromatin and centromeres) and preexisting microtubules.Citation1 Central to this, the Ran system controls microtubule physiology through the regulation of the interaction of different SAFs with importins. Thus, SAF function is inhibited by importin binding, and during mitosis RanGTP promotes SAF release around the chromosomes where it is produced by the chromatin-bound nucleotide exchange factor RCC1.Citation1
The Vernos Lab has long been interested in understanding how different SAFs (such as the multifunctional protein TPX2) are controlled by Ran and previously reported MCRS1 as a novel SAF regulated by this pathway. This protein is involved in chromatin-dependent microtubule stabilization and caps the minus ends of K-fibers, the microtubule bundles that connect chromosomes to the spindle poles.Citation2 K-fibers microtubules are more stable than other fiber microtubules, and MCRS1, localized at microtubules minus ends by the NSL complex component KANSL3, prevents K-fiber microtubule depolymerization by the kinesin MCAK and other MT depolymerases, while also regulating microtubule minus end dynamics.Citation3
The function of most mitotic proteins is controlled to a greater or lesser degree by phosphorylation, with their activities, localization and/or binding partners altered by the incorporation of one or more phosphate groups. In this context, phosphorylation can relay spatial or temporal signals and interrelate in different ways with other signaling systems such as that of Ran. SAFs are not an exception to this, and now Meunier, Vernos and collaborators continuing their previous work show that MCRS1 function is also regulated by phosphorylation. In their recent article in Cell CycleCitation4 they uncover MCRS1 as a novel mitotic substrate for Aurora A kinase and show that MCRS1 phosphorylation at specific sites control its activity at K-fibers. Specifically, the authors show that MCRS1 coimmunoprecipitates with Aurora A and that the kinase phosphorylates it at different sites, among them Ser35/Ser36, 2 sites that are also modified in vivo in mitotic cells. Ser35/Ser36 are not necessary for the interaction with known MCRS1 partners or the normal localization of MCRS1 in mitotic cells, but are needed for the normal function of the protein during mitosis, with the non-phosphorylable mutant not being able to rescue the mitotic abnormalities that arise upon endogenous MCRS1 downregulation. What is the function of the studied phosphorylations? Discarded a clear-cut effect on chromatin-induced MT organization, the authors investigate whether the modification of Ser35/Ser36 could be regulating K-fiber organization. Indeed, phosphonull MCRS1 mutants failed to rescue the effect of MCRS1 downregulation on K-fibers (e.g. fiber shortening and a concomitant decrease of stability). This mutants are able to effectively rescue chromatin-dependent microtubule organization, thus suggesting that MCRS1 may have distinct functions during chromatin-induced microtubule formation and their subsequent incorporation into K-fibers, and that phosphorylation controls a late MCRS1 role on K-fiber assembly and dynamics, possibly around centrosomes, where active Aurora A is concentrated.
Surprisingly phosphomimetic substitutions of MCRS1 Ser35/Ser36 fail to rescue both chromatin-dependent microtubule organization and K-fiber function, and even behave as dominant negative mutations. This could be the result, as the authors suggest, of an adverse effect of phosphorylation on MCRS1 function in one or both processes (although the possibility of the substitutions not being bona fide phosphomimetic mutations cannot be totally excluded).
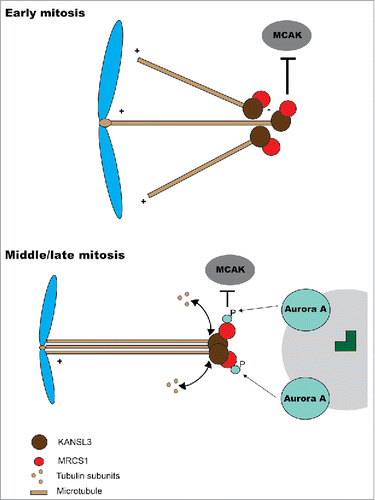
This interesting work deserves to be expanded with the aim of dissecting the molecular details of MCRS1 different functions and how phosphorylation of Ser35/Se36 and other residues modulate them. Possible effects of phosphorylation on protein localization during different stages of mitosis (e.g., prometaphase, during chromatin-induced aster formation) should also be studied, as different MCRS1 mutant forms seem to have distinct localizations during MT-regrowth experiments but not at metaphase. Overall, available data indicates that MCRS1 is, quite possibly as other SAFs, regulated both by the Ran system and by different phosphorylation events. Based on this, Meunier and collaborators propose a mechanism in which during early mitosis MCRS1, freed from importin by RanGTP, acts capping K-fibers that emerge from chromosomes and prevents their depolymerization. Once K-fibers reach spindle poles, MCRS1 is phosphorylated by Aurora A, and this affects MCRS1 activity, thus switching its function toward the control of K-fiber length and dynamics (). A fine example of how 2 different signaling systems control an important process in an interrelated manner.
Figure 1. Model for MCRS1 function. At early mitosis MCRS1 (top) interacts with KANSL3 at minus end of microtubules nucleated from chromosomes. This localization at minus ends prevents microtubule depolimeryzation by the action of MCAK and other depolymerases. At later stages in mitosis (bottom), the minus ends of K-fibers are closer to the poles and MCRS1 becomes phosphorylated by Aurora A kinase. This phosphorylation affects MCRS1 activity but not its localization.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Reference
- Cavazza T, Vernos I. The RanGTP Pathway: From Nucleo-Cytoplasmic Transport to Spindle Assembly and Beyond. Front Cell Dev Biol 2015; 3:82; PMID:26793706; http://dx.doi.org/10.3389/fcell.2015.00082
- Meunier S, Vernos I. K-fibre minus ends are stabilized by a RanGTP-dependent mechanism essential for functional spindle assembly. Nat Cell Biol 2011; 13(12):1406-14; PMID:22081094; http://dx.doi.org/10.1038/ncb2372
- Meunier S, Shvedunova M, Van Nguyen N, Avila L, Vernos I, Akhtar A. An epigenetic regulator emerges as microtubule minus-end binding and stabilizing factor in mitosis. Nat Commun 2015; 6:7889; PMID:26243146; http://dx.doi.org/10.1038/ncomms8889
- Meunier S, Timón K, Vernos I. Aurora-A regulates MCRS1 function during mitosis. Cell Cycle 2016; 15(3):1779-86; PMID:27192185; http://dx.doi.org/10.1080/15384101.2016.1187342
