One of the cellular defensive mechanisms against proteotoxic stress is the evolutionarily conserved proteotoxic stress response (PSR) or heat-shock response (HSR). Through up-regulation of molecular chaperones or heat-shock proteins (HSPs), the PSR empowers cells to repair and/or dispose of misfolded and aggregated proteins,Citation1 thereby enduring stressful environments. Beyond being vital to stress resistance, the PSR has been implicated in a wide array of human pathological conditions, including cancer and neurodegenerative disorders.Citation1 The master transcriptional regulator of the PSR is heat shock factor 1 (HSF1). Unsurprisingly, the HSF1-mediated PSR plays a pivotal role in preserving cellular proteome homeostasis or proteostasis.Citation2
Two central factors governing cellular proteostasis are protein quantity and quality. While HSPs ensure the quality of cellular proteome, the mammalian target of rapamycin complex 1 (mTORC1) critically controls protein quantity. Stimulated by growth factors and nutrients, mTORC1 prompts protein translation by phosphorylating both the ribosomal S6 kinase (S6K) and the eukaryotic translation initiation factor 4E binding protein 1 (EIF4EBP1).Citation3 By contrast, a diversity of insults, including genotoxic, hypoxic, metabolic, and oxidative stress, all suppress mTORC1 through activation of either the AMP-activated protein kinase (AMPK) or the tuberous sclerosis complex 1/2 (TSC1/2).Citation3 Despite its crucial role in controlling protein biosynthesis, surprisingly, little is known of how mTORC1 senses and responds to proteotoxic stress.
JNK is a component and negative regulator of mTORC1
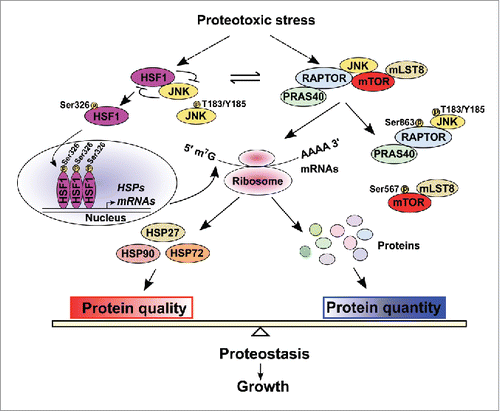
Recent findings start shedding light on this question. Our studies revealed that proteotoxic stress activates c-JUN N-terminal kinase (JNK) to suppress mTORC1 and its mediated translation.Citation4 Mechanistically, our studies unveiled a constitutive association of JNK with mTORC1 even under normal growth conditions.Citation4 Upon activation, JNK directly phosphorylates both mTOR at Ser567 and regulatory-associated protein of mTOR (RAPTOR) at Ser863.Citation4 These modifications cause selective exclusion of mTOR and GβL/mLST8 from mTORC1.Citation4 By contrast, the associations among JNK, RAPTOR, and PRAS40 remain unaffected. Congruent with its impact on mTORC1, our studies collectively demonstrated JNK as a negative regulator of global protein synthesis, cell size, and whole-body lean mass.Citation4
HSF1 de-represses mTORC1 independently of transcription
Importantly, our studies further uncovered that the JNK-mediated mTORC1 suppression is finely restrained by HSF1, a key player in combating proteotoxic stress. Through physical interactions, HSF1 sequesters JNK away from mTORC1,Citation4 thereby relieving the suppression imposed by JNK. Highlighting a transcription-independent nature, HSF1 mutants lacking transcriptional activity also segregate JNK from and stimulate mTORC1.Citation4 This mode of action is sharply contrasting with the widely recognized transcriptional activity of HSF1. Congruent with its effect on mTORC1, HSF1 positively regulates protein translation, cell size, and body lean mass.Citation4
Biological consequences of the HSF1-mTORC1 interplay
First, mTORC1 acts as a key cellular sensor of proteotoxic stress. Thus, in addition to energetic, genotoxic, and oxidative stress, mTORC1 is inherently composed to perceive proteotoxic stress. Upon proteotoxic challenge, cells must mitigate global protein translation, in part through mTORC1 inhibition. This adaptive reaction serves as a necessary step to alleviate proteomic burden and further recover from the stress.
Second, HSF1 governs the PSR both transcriptionally and translationally. It has been widely believed that HSF1 regulates the PSR predominantly at the transcription level. However, our findings now illuminate that by averting hyper-activation of JNK and subsequent hyper-repression of mTORC1, HSF1 enables robust translation of HSP mRNAs highly induced by HSF1 under proteotoxic stress. This translational regulation is crucial, as without sufficient translational capacity massively transcribed HSP mRNAs would not be converted into functional chaperones that are required to resolve proteotoxic stress.
Third, by orchestrating the protein quantity- and quality-control machineries, HSF1 warrants vigorous growth. Canonically, HSF1 has been regarded to primarily guard protein quality via the PSR. Now, the HSF1-mTOTC1 interaction pinpoints a previously unrecognized role of HSF1 in controlling protein quantity. HSF1 deficiency not only impairs protein quality due to depleted chaperoning capacity, as expected, but also diminishes protein quantity due to unexpected mTORC1 inhibition. Intriguingly, deficiency in the protein quality-control machinery causatively impairs the protein quantity-control machinery, retarding cellular and organismal growth. Thus, our findings reveal that HSF1, by strictly coordinating protein quantity and quality, acts as a key determinant of growth ().
Outstanding questions
Despite these exciting findings, many important questions remain outstanding. Here are just a few examples. Our studies revealed that diverse proteotoxic stressors, including heat shock, amino acid analogs, chaperone inhibitors, and proteasome blockers, all trigger JNK activation and mTORC1 suppression. Nonetheless, the very signal commonly activating JNK under these various conditions remains to be determined. Furthermore, in addition to proteotoxic stressors, JNK can also be activated by a myriad of other environmental cues, including UV and γ irradiation, inflammatory cytokines, oxidative stress, apoptotic stimuli, as well as osmotic shock.Citation5 However, it remains unknown whether these environmental cues also impact mTORC1 via JNK. Moreover, despite its importance in growth control, it remains unclear how widespread the transcription-independent action of HSF1 is in biology. Lastly, whereas amounting evidence has unequivocally indicated HSF1 as a powerful pro-oncogenic factor,Citation6,7 it still remains unaddressed whether HSF1 employs the same mechanism to promote malignant growth, a question that may have im-portant implications for anti-cancer therapies.
Figure 1. HSF1 Balances protein quantity and quality. On the one hand, HSF1 maintains mTORC1 activity through suppression and sequestration of JNK, thereby controlling translation of proteins including stress-induced HSPs. On the other hand, HSF1, in response to proteotoxic stress, markedly up-regulates HSPs expression, thereby ensuring protein quality. By orchestrating protein quantity and quality, HSF1 preserves proteostasis and empowers growth.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev 2008; 22:1427-38; PMID:18519635; http://dx.doi.org/10.1101/gad.1657108
- Vihervaara A, Sistonen L. HSF1 at a glance. J Cell Sci 2014; 127:261-6; PMID:24421309; http://dx.doi.org/10.1242/jcs.132605
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 2010; 40:310-22; PMID:20965424; http://dx.doi.org/10.1016/j.molcel.2010.09.026
- Su KH, Cao J, Tang Z, Dai S, He Y, Sampson SB, Benjamin IJ, Dai C. HSF1 critically attunes proteotoxic stress sensing by mTORC1 to combat stress and promote growth. Nat Cell Biol 2016; 18:527-39; PMID:27043084; http://dx.doi.org/10.1038/ncb3335
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000; 103:239-52; PMID:11057897; http://dx.doi.org/10.1016/S0092-8674(00)00116-1
- Tang Z, Dai S, He Y, Doty RA, Shultz LD, Sampson SB, Dai C. MEK guards proteome stability and inhibits tumor-suppressive amyloidogenesis via HSF1. Cell 2015; 160:729-44; PMID:25679764; http://dx.doi.org/10.1016/j.cell.2015.01.028
- Dai C, Sampson SB. HSF1: guardian of proteostasis in cancer. Trends Cell Biol 2016; 26:17-28; PMID:26597576; http://dx.doi.org/10.1016/j.tcb.2015.10.011
