Many previous studies have shown that many health benefits in calorie restriction (CR) are attributed to the down-regulation of the mammalian target of rapamycin complex 1 (mTORC1) and the up-regulation of NAD dependent protein deacetylase SIRT1.Citation1,2 In the intestine, CR was reported to increase the number of intestinal stem cells (ISCs) by reducing mTORC1 signaling in Paneth or niche cells, thereby inducing the bone stromal antigen 1 (Bst-1), an ectoenzyme that produces the paracrine factor cyclic ADP ribose (cADPR).Citation3 cADPR secreted by Paneth cells then signals the division of intestinal stem cells (ISCs) to favor self-renewal instead of differentiation resulting in an increase in the number of ISCs.
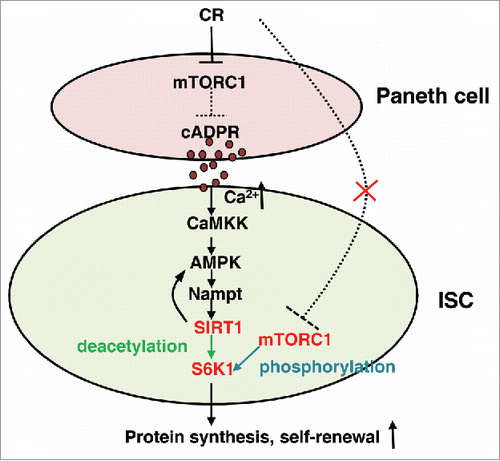
Unexpectedly, we recently reported that mTORC1 activity in ISCs is up-regulated during CR, resulting in an increase in protein synthesis in ISCs and an increase in their cell number.Citation4 We traced the signaling pathway in ISCs by using genetic and pharmacological interventions in mice, isolated crypts, and co-cultures of purified ISCs and Paneth cells. These studies revealed that ISCs respond to cADPR via 1) Ca++ signaling, 2) AMPK/SIRT1 activation, and 3) S6K1 activation by mTORC1 phosphorylation (). The relevant links between these steps are the activation of AMPK by the calcium dependent CaMKK, the activation of Nampt synthesis, NAD production and SIRT1 by AMPK, and the deacetylation of S6K1 by SIRT1, which promotes its phosphorylation by mTORC1. This signal-response mechanism helps mTORC1 signaling to increase in ISCs during CR, even as it is turned down in differentiated cells in the intestinal villi, including Paneth cells. Knocking out SIRT1 in ISCs prevented the response to signaling by CR Paneth cells, while knocking out SIRT1 in Paneth cells had no effect. SIRT1 gain of function in ad libitum fed mice gave rise to ISC expansion and villi contraction similar to CR mice, suggesting that SIRT1 activation in ISCs is not only necessary but sufficient to favor their expansion via activation of mTORC1/SIRT1 signaling. Consistent with this model, mTOR inhibitor rapamycin treatment inhibits mTORC1 and prevents ISC expansion by CR in mice.
Figure 1. The scheme of cADPR induced stem cell proliferation. cADPR activate AMPK/Nampt/SIRT1 loop via the activation of Ca2+, CaMKK. S6K1 deacetylation by SIRT1 promotes S6K1 phosphorylation by mTORC1, resulting in the increase of protein synthesis and self-renewal in ISC. Unexpectedly, mTORC1 in ISCs does not sense CR directly, unlike Paneth cells.
Generally mTORC1 activity can be suppressed via the downregulation of insulin-PI3K-AKT signaling or AMPK activation during calorie restriction. However insulin-PI3K-AKT signaling is not altered in ISCs during CR. In addition, WNT/β-catenin signaling, known to drive cell division in ISCs, is not affected by CR.
Surprisingly, activated AMPK does not phosphorylate the mTORC1 inhibitor TSC2 or the TOR partner raptor to suppress mTORC1 activity in ISCs. More generally, CR does not suppress the assembly of mTORC1 complex, whereas rapamycin does, as expected. ISCs thus appear to be insulated from the direct sensing of CR, and instead respond only to the cADPR signal emitted from Paneth cells. This unusual situation would allow close communication between ISCs and Paneth cells, and enable the upregulation of the mTORC1/SIRT1 axis in ISCs during CR.
In summary, down-regulation of mTORC1 in Paneth cells is necessary for sending the cADPR signal, whereas up-regulation of mTORC1 in ISCs is necessary to respond and drive the increase in cell number. The opposite regulation of mTORC1 in different cell types raises a complication in utilizing rapamycin to mimic the salutary effects of CR globally in animals. It might be possible to find a dose of rapamycin that would suppress mTORC1 enough to induce the secretion of cADPR in Paneth cells but still leave sufficient activity in ISCs to respond. However, we actually found our 2 different regimens of rapamycin treatment (oral rapamycin gavage and eRapa food) suppress the expansion of ISCs in CR mice, but do not alter the population of ISCs in ad libitum fed mice. On the other hand, SIRT1 activation or NAD supplementation by NAD precursors such as NR and NMNCitation5 may be able to effectively mimic CR in the gut. In particular, NAD supplementation would not only foster cADPR synthesis in Paneth cells but also activate SIRT1 in ISCs, thereby elevating mTORC1 signaling and increasing their cell number. It will be interesting to test whether ISC maintenance during normal aging also requires the mTORC1/SIRT1 axis, and, if so, whether NR or NMN can rescue any aging-induced loss of ISCs.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274-93; PMID:22500797; http://dx.doi.org/10.1016/j.cell.2012.03.017
- Guarente L. Calorie restriction and sirtuins revisited. Genes Dev 2013; 27:2072-85; PMID:24115767; http://dx.doi.org/10.1101/gad.227439.113
- Yilmatz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 2012; 486:490-5; PMID:22722868; http://dx.doi.org/10.1038/nature11163
- Igarashi M, Guarente L. mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 2016; 66:436-50. PMID:27345368; http://dx.doi.org/10.1016/j.cell.2016.05.044
- Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol 2014;24:464-71. PMID:24786309; http://dx.doi.org/10.1016/j.tcb.2014.04.002
