Fasting, meaning the total absence of caloric uptake coupled to ad libitum access to water, is one strategy that may reduce tumor growth in preclinical models, in mice. Thus, depending on the cancer model that is investigated, fasting decreases tumor growth either on its ownCitation1 or in combination with cytotoxic chemotherapies.Citation2 Initial investigations focused on the idea that fasting would mediate anticancer effects either due to a reduction in circulating nutrients (like glucose or amino acids) or due to a drop in trophic hormones (such as insulin and insulin growth factor-1, IGF1), hence compromising the survival and proliferation of malignant cells.Citation1,2 In apparent support of this interpretation, it was found that tumors that constitutively activate trophic signaling by oncogenic mutation of class 1 phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α (PIK3CA) do not reduce their growth upon fasting.Citation1
Most if not all cytotoxic chemotherapeutics have been developed based on the assumption that they would act like antibiotics and hence arrest the cell cycle and/or kill cancer cells in a selective fashion. However, in recent years it has turned out that chemotherapy is particularly efficient if it succeeds in stressing and killing malignant cells in a way that elicits a specific immune response against tumor-associated antigens. Indeed, in preclinical models, depletion of cytotoxic T lymphocytes (CTL) is sufficient to completely abolish tumor growth reduction induced by anthracyclines, cyclophosphamide or oxaliplatin.Citation3,4 These chemotherapeutics trigger immunogenic cell death (ICD). ICD is characterized by a series of premortem stress signal that alert cells from the innate immune system, in particular dendritic cell (DC) precursors, that invade the tumor bed, place themselves in the proximity of dying cancer cells, take up tumor-associated antigens and present them to CTL (Vacchelli 2015). One of the hallmark characteristics of ICD is autophagy, which facilitates the release of immunostimulatory adenosine triphosphate (ATP) over that of immunosuppressive adenosine, hence favoring the chemotactic attraction of DC precursors into the tumor bed and reducing that of immunosuppressive regulatory T cells (Treg). Autophagy-deficient cancers are characterized by a reduced DC infiltration as well an unfavorable CTL/Treg ratio post-chemotherapy and hence do not elicit a growth-inhibitory anticancer immune response.Citation3
The combination of ICD-inducing chemotherapies with starvation yields superior tumor growth-inhibitory effects that were fully immune dependent.Citation5,6 Thus, the combination of starvation plus chemotherapy (with anthracyclines or oxaliplatin) reduced cancer progression more than each of these interventions alone, and growth reduction disappeared when mice were lacking T lymphocytes. Tumors from which essential elements of the autophagic machinery (such as Atg5 or Atg7) were depleted failed to respond to the combination regimen of starvation and chemotherapy.Citation5 Altogether, these results underscore the importance of the link between autophagy occurring in malignant cells and subsequent activation of anticancer immunosurveillance.Citation3
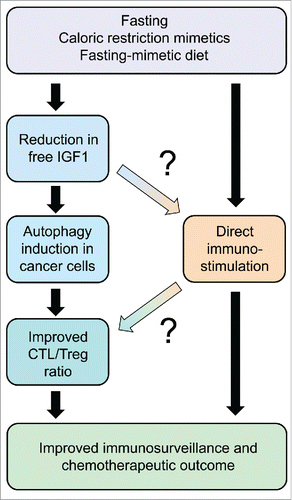
In mice, a 48-hour starvation period causes a drastic reduction in body weight (by approximately 20%) that is, however, fully reversible. Driven by the consideration that such a weight loss might create subjective discomfort, we and others have developed alternative strategies to avoid full-fledged starvation. Valter Longo and colleagues developed a hypocaloric “fasting-mimetic diet” that causes a moderate reduction of body weight, yet an important fall in free circulating IGF-1 levels, yielding immune-dependent improvement of chemotherapeutic outcome.Citation6 We used an alternative strategy consisting in the artificial induction of autophagy with non-immunosuppressive agents. Most of these agents fall into the category of “caloric restriction mimetics” (CRMs), meaning that they directly induce one of the biochemical hallmarks of cellular starvation, namely a reduction in cytoplasmic protein acetylation.Citation7 This effect is achieved by inhibition of the supply of cytosolic acetyl-coenzyme A (due to the inhibition of ATP citrate lyase, for instance by hydroxycitrate), by the inhibition of the acetyl transferase activity of EP300 (for instance by spermidine) or by the activation of the deacetylase activity of sirtuin-1 (for instance by resveratrol). Indeed, CRMs including hydroxycitrate, spermidine and resveratrol all induced autophagy in cancer cells and improved anticancer immune response with consequent tumor growth reduction if they were combined with immunogenic chemotherapeuticsCitation5 Of note, these effects were not linked to any significant weight loss, yet disappeared upon suppression of autophagy in malignant cells or upon removal of CTL from the system. Moreover, they were coupled to a favorable shift in the CTL/Treg ratio within the tumor bed indicating improved local immune response. Epistatic experiments indicate that CRMs stimulate anticancer immunosurveillance via the depletion of Tregs.Citation5 This Treg depletion fully depends on the autophagy competence of cancer cells and can be reversed by restoring free IGF1 levels to the normal level (which also inhibits autophagy induction by CRMs), underscoring the existence of an axis that links fasting (or CRMs) to IGF1 reduction, consequent autophagy induction in cancer cells and local immunostimulation. It has not been yet explored whether CRMs have immunostimulatory effect by a direct (cancer cell-independent) effect on immune effectors. However, FMD turned out to increase the frequency of common T lymphocyte precursors,Citation6 suggesting that such an effect might be therapeutically relevant (). Irrespective of these mechanistic incognita, it appears that fasting and its substitutes (CRMs and FMD) may improve anticancer immunosurveillance in preclinical models.
Figure 1. Schematic overview of the anticancer effects of fasting and its substitutes, namely pharmacological agents that mimic the biochemical effects of starvation (caloric restriction mimetics) or a specific formulation of nutrient that mimics fasting (fasting mimicking diet). Note that the scheme constitutes a working hypothesis. Connections that appear particularly uncertain are marked with question marks. CTL, cytotoxic T lymphocyte; Treg, regulatory T cell.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature 2009; 458:725-31; PMID:19279572; http://dx.doi.org/10.1038/nature07782
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med 2012; 4:124ra27.; PMID:22323820; http://dx.doi.org/10.1126/scitranslmed.3003293
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011; 334:1573-7.; PMID:22174255; http://dx.doi.org/10.1126/science.1208347
- Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 2015; 350:972-8.; PMID:26516201; http://dx.doi.org/10.1126/science.aad0779
- Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, Levesque S, Castoldi F, Jacquelot N, Yamazaki T, et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell 2016; 30:147-60.; PMID:27411589; http://dx.doi.org/10.1016/j.ccell.2016.05.016
- Di Biase S, Lee C, Brandhorst S, Manes B, Buono R, Cheng CW, Cacciottolo M, Martin-Montalvo A, de Cabo R, Wei M, et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 2016; 30:136-46.; PMID:27411588; http://dx.doi.org/10.1016/j.ccell.2016.06.005
- Marino G, Pietrocola F, Eisenberg T, Kong Y, Malik SA, Andryushkova A, Schroeder S, Pendl T, Harger A, Niso-Santano M, et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell 2014; 53:710-25; PMID:24560926; http://dx.doi.org/10.1016/j.molcel.2014.01.016
