DNA double-strand breaks (DSBs) can arise through exposure to exogenous DNA damaging agents such as ionizing radiation, as well as through endogenous means; for example, via DNA replication fork collapse. Irrespective of the source, the physical severing of the sugar-phosphate backbone represents an acute threat to organismal viability and genome stability. Hanahan and Weinberg describe genome instability and mutation as an enabling characteristic of cancer.Citation1 Indeed, chromosome structural rearrangements, which are pervasive in cancer, invariably arise from DSB repair gone awry. To deal with this threat, all cells have evolved 2 principal means to repair DSBs: homologous recombination (HR) and nonhomologous DNA end joining (NHEJ). HR is predominantly a conservative and error-free process, employing a homologous template, usually the sister chromatid, to repair the break. RAD51, the eukaryotic ortholog of bacterial RecA, is the major HR protein. Conversely, NHEJ is typically error-prone, and rejoins the break without regard for the state of the ends, often resulting in loss of nucleotides or the re-joining of noncontiguous ends. Exemplifying the importance of HR, many key tumor suppressor genes encode central HR players, e.g. BRCA1 and BRCA2. Furthermore, several genetic diseases characterized by increased cancer risk are caused by mutations in HR genes, one example of which is Fanconi anemia (FA).
FA is clinically characterized by congenital defects, bone marrow failure, and increased cancer risk.Citation2 FA is caused by mutation of any one of 20 known genes, which encode proteins that function cooperatively in the FA-BRCA pathway to promote HR.Citation3 The molecular links between FA and HR are an area of active investigation. Evidence presented in this volume of Cell Cycle points to a novel noncanonical connection between enzymes involved in the major regulatory step of the FA-BRCA pathway and a key HR effector.Citation4 This regulatory step is the site-specific monoubiquitination of the FANCD2 and FANCI proteins. The E2 ubiquitin-conjugating enzyme FANCT/UBE2T and the E3 ubiquitin ligase FANCL catalyze the forward step of this reaction. The reverse step - deubiquitination - is catalyzed by the USP1 deubiquitinating enzyme (DUB) and its heterodimeric binding partner UAF1.Citation5,6
In a large-scale global proteomic screen of DUB-interacting networks, Sowa et al. previously determined that the USP1-UAF1 heterodimer interacts with the RAD51AP1 protein.Citation7 RAD51AP1 is a vertebrate specific accessory factor for RAD51 that promotes the assembly of the synaptic complex and D (displacement)-loop, key HR intermediate structures (). However, the functional significance of this interaction was not examined. In the accompanying Cell Cycle manuscript, Cukras et al. have tackled this important question.Citation4 The authors verified this interaction and, by depleting UAF1 using siRNA, established that the interaction between USP1 and RAD51AP1 is UAF1-dependent. The authors also established that the UAF1 WD40 repeats as well as its SUMO-like domains (SLDs) are necessary for RAD51AP1 binding. Previous studies had demonstrated that USP1 regulates the stability of the ID (inhibitor of DNA binding) proteins. Similarly, Cukras et al. show that depletion of USP1 or UAF1 leads to destabilization of RAD51AP1.
Figure 1. Speculative schematic of the role of the USP1-UAF1-RAD51AP1 complex in HR. UAF1 binds to USP1through its WD40 domain, and RAD51AP1 through its SLD1/2 domains. In the absence of either UAF1 or USP1, RAD51AP1 is degraded by the proteasome. Following RAD51 nucleofilament formation, RAD51AP1 is required for synaptic complex and D-loop formation. This is promoted by the presence of UAF1, however the role of USP1 in this process remains unclear. On the right side of this figure, USP1 is depicted in gray font to signify its uncertain role in this process.
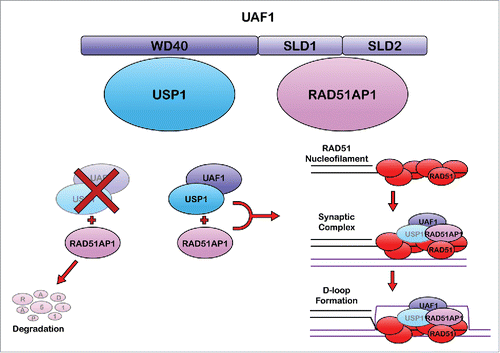
Cukras et al. next sought to map the region of RAD51AP1 that binds to UAF1. Serial truncations and mutagenesis analysis established that residues D133-L137 are required for efficient RAD51AP1-UAF1 binding. Accordingly, deletion of this UAF1 binding region (ΔDYLDL) resulted in decreased RAD51AP1 stability, supporting the theory that USP1-UAF1-RAD51AP1 form a stable protein complex. Interestingly, mutation of RAD51AP1 K139, previously shown to be a site of ubiquitination, did not affect interaction with UAF1. To explore the functional significance of the RAD51AP1-UAF1 interaction, Cukras et al. expressed wild type or RAD51AP1-ΔDYLDL in U2OS cells depleted of endogenous RAD51AP1. In contrast to wild type RAD51AP1, the ΔDYLDL mutant failed to correct cellular ICL sensitivity. Furthermore, RAD51AP1-ΔDYLDL expressing cells exhibited persistent DNA damage-inducible RAD51 nuclear foci, suggesting that the USP1-UAF1-RAD51AP1 complex may promote the efficient and timely resolution of a key HR intermediate structure.
A recent complementary study in Cell Reports by Liang et al. provides further insight into the functional significance of the RAD51AP1-UAF1 interaction.Citation8 Similar to Cukras et al., Liang et al. establish that the UAF1 SLDs mediate interaction with RAD51AP1. While mutation of these SLDs compromises interaction with RAD51AP1, these mutants are proficient for interaction with USP1 and stimulation of its DUB activity toward FANCD2. Importantly, Liang et al. also establish that UAF1 alone stimulates the ability of RAD51AP1 to promote synaptic complex and D-loop formation in vitro, and this stimulation depends on the formation of the RAD51AP1-UAF1 complex. These assays indicate that UAF1-stimulated RAD51AP1 activity is largely USP1-independent. While Cukras et al. clearly show that USP1 forms a complex with UAF1 and RAD51AP1, a role for enzymatic deubiquitination has not been established. Taken together, these studies reveal a novel and critical function for UAF1 in promoting HR that appears to be independent of USP1 deubiquitinating activity. However, it remains to be determined how RAD51AP1 is removed from RAD51 nucleoprotein filaments enabling the dissolution of HR intermediates - ubiquitination remains a plausible mechanism. In conclusion, these studies uncover important mechanistic insight into the molecular biology of HR and FA and suggest the existence of more FA genes linked to the regulation of RAD51 function.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Institutes of Health/National Heart, Lung and Blood Institute grant R01HL101977 to NGH, and Rhode Island IDeA Network of Biomedical Research Excellence (RI-INBRE) grant P20GM103430 from the National Institute of General Medical Sciences.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Fund FAR. Fanconi anemia: guidelines for diagnosis and management. 4th ed. Eugene, OR: Fanconi Anemia Research Fund; 2014; 1-429.
- Walden H, Deans AJ. The Fanconi anemia DNA repair pathway: structural and functional insights into a complex disorder. Annu Rev Biophys 2014; 43:257-78; PMID:24773018; http://dx.doi.org/10.1146/annurev-biophys-051013-022737
- Cukras S, Lee E, Palumbo P, Benavidez P, Moldovan GL, Kee Y. The USP1-UAF1 complex interacts with RAD51AP1 to promote homologous recombination repair. Cell Cycle 2016; 15(19):2636-2646; PMID:27463890; http://dx.doi.org/10.1080/15384101.2016.1209613
- Cohn MA, Kowal P, Yang K, Haas W, Huang TT, Gygi SP, D'Andrea AD. A UAF1-containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol Cell 2007; 28:786-97; PMID:18082604; http://dx.doi.org/10.1016/j.molcel.2007.09.031
- Nijman SM, Huang TT, Dirac AM, Brummelkamp TR, Kerkhoven RM, D'Andrea AD, Bernards R. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol Cell 2005; 17:331-9; PMID:15694335; http://dx.doi.org/10.1016/j.molcel.2005.01.008
- Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell 2009; 138:389-403; PMID:19615732; http://dx.doi.org/10.1016/j.cell.2009.04.042
- Liang F, Longerich S, Miller AS, Tang C, Buzovetsky O, Xiong Y, Maranon DG, Wiese C, Kupfer GM, Sung P. Promotion of RAD51-mediated homologous DNA pairing by the RAD51AP1-UAF1 complex. Cell Rep 2016; 15:2118-26; PMID:27239033; http://dx.doi.org/10.1016/j.celrep.2016.05.007
