ABSTRACT
Exposure of renal cells to high glucose (HG) during diabetes has been recently proposed to be involved in renal injury. In the present study, we investigated a potential mechanism by which AICAR treatment regulates the DNA repair enzyme, 8-oxoG-DNA glycosylase (OGG1) in renal proximal tubular mouse cells exposed to HG and in kidney of db/db mice. Cells treated with HG for 2 days show inhibition in OGG1 promoter activity as well as OGG1 and Nrf2 protein expression. In addition, activation of AMPK by AICAR resulted in an increase raptor phosphorylation at Ser792 and leads to increase the promoter activity of OGG1 through upregulation of Nrf2. Downregulation of AMPK by DN-AMPK and raptor and Nrf2 by siRNA resulted in significant decease in promoter activity and protein expression of OGG1. On the other hand, downregulation of Akt by DN-Akt and rictor by siRNA resulted in significant increase in promoter activity and protein expression of Nrf2 and OGG1. Moreover, gel shift analysis shows reduction of Nrf2 binding to OGG1 promoter in cells treated with HG while cells treated with AICAR reversed the effect of HG. Furthermore, db/db mice treated with AICAR show significant increased in AMPK and raptor phosphroylation as well as OGG1 and Nrf2 protein expression that associated with significant decrease in oxidative DNA damage (8-oxodG) compared to non-treated mice. In summary, our data provide a novel protective mechanism by which AICAR prevents renal cell damage in diabetes and the consequence complications of hyperglycemia with a specific focus on nephropathy.
Introduction
Hyperglycemia is the main metabolic abnormality in diabetes that is able to stimulate a generation of reactive oxygen species (ROS). Oxidative stress leads to protein, lipid, and DNA modifications that cause cellular dysfunction and contribute to the pathogenesis of complications of diabetes, including diabetic nephropathy.Citation1,2 Damage most likely occurs when the endogenous antioxidant network and DNA repair systems are overwhelmed.Citation3,4 However, it is essential for the cell to repair DNA damage induced by oxidants. 8-Oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) is a sensitive marker of ROS–induced DNA damage.Citation5 There is an increase in 8-oxodG levels in tissue of diabetic rats and in the urine of patients with type 1 and type 2 diabetes with the levels being significantly higher in patients with albuminuria or with other diabetic complications.Citation6 DNA repair enzyme, 8-oxoG-DNA glycosylase (OGG1) expression has important functional consequences, compromising the ability of cells to repair the DNA damage 8-oxodG. The steady-state level of 8-oxodG in DNA reflects its rate of generation and of repair.Citation7,8 The transcription factor NFE2-related factor 2 (Nrf2) plays an important role in the regulation of cellular detoxification responses upon exposure of cells to oxidative stress.Citation9 Nrf2 is a basic-region leucine zipper transcription factor that regulates basal activity and inducible expression of a battery of environmental stress response genes.Citation10 In addition, Nrf2 regulates the transcription of a plethora of cytoprotective genes including those encoding antioxidant and phase II-detoxifying enzymes.Citation9
AMP-activated protein kinase (AMPK) has been recognized as an important upstream signaling intermediate intimately involved in the downregulation of the mTOR pathway.Citation11 In addition, AMPK has been described as a cellular fuel gauge, increasing its kinase activity in response to decreases in cellular energy charge.Citation12 The function of activated AMPK is to decrease the rate of cellular processes that require energy, while at the same time increasing the rate of energy-generating processes to return the cellular energy charge to a more replete state.Citation13 AMPK activation by metformin or 5-aminoimidazole-4-carboxamide-1β-riboside (AICAR) suggests that pharmacological activation of this kinase may be beneficial in the treatment of type II diabetes.Citation14 AMPK is a physiological cellular energy sensor that is activated by phosphorylation at Thr172 in response to changes in cellular ATP levels.Citation15,16
The potential therapeutic effects of AMPK activation on glucose metabolism suggest that activation of AMPK may also have favorable effects on disorders of lipid cell metabolisms.Citation17 While AICAR effectively activates AMPK, the mechanism by which AICAR can inhibit the formation and progression of diabetes complications through AMPK/NRF2/OGG1/mTOR pathway is not fully understood. In the present study, we investigated the potential role of AICAR in the regulation of the DNA repair enzyme OGG1 in renal proximal tubular cells and in kidney of db/db mice to understand the mechanism by which AICAR prevents kidney damage in diabetes.
Results
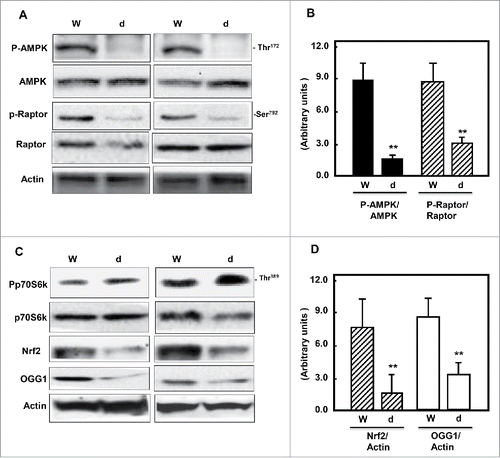
Decreased AMPK activity is associated with downregulation of Nrf2 and OGG1 expression in db/db mice
AMPK is a fuel-sensing enzyme that is activated by a decrease in the cellular energy state, as reflected by an increased AMP/ATP ratio. To investigate the effect of hyperglycemia on AMPK/mTOR pathway, kidney cortex homogenates of wild type and db/db mice were analyzed by immunoblotting. Cellular levels of AMPK phosphorylation at Thr172 and raptor at Ser92 showed a significant decreased in kidney cortex of db/db mice compared to wild type mice (). In addition, Phosphorylation of p70S6K (a marker of mTORC1) was significantly increased in kidney tissue homogenates of diabetic animals compared to wild type animals. The decrease phosphorylation of AMPK and activation of mTORC1/raptor is associated with decrease protein expression of Nrf2 and downregulation protein expression of OGG1 in diabetic mice compared to wild type mice ).
Figure 1. Diabetes inhibits AMPK and decreases mTORC1, Nrf2 and OGG1 protein expression. (A & C) Representative Immunoblot analysis shows a significant decrease in AMPK phosphorylation at Thr172 and raptor at Ser792 (marker of mTORC1 activation) in kidney cortex of db/db mice (d) compared to wild type mice (W). Inactivation of AMPK and activation of mTORC1 resulted in significant decrease of Nrf2 and OGG1 protein expression in kidney cortex of diabetic compared to wild type mice. Actin was used as a loading control. (B & D) Histograms represent means ± SE (n = 4). Significant difference from wild type is indicated by *P < 0.01.
AICAR improves kidney parameters in diabetic mice
The pharmacological activators of AMPK such as AICAR and metformin inhibit the cell growth in diabetes. To demonstrate whether AICAR-treatment improves kidney parameters of diabetes, db/db mice were treated with AICAR at 2 mg/kg body weight for 5 d/week for 4 weeks. AICAR showed significant effect on reducing blood glucose and decreasing proteinuria, albuminuria and creatinine in urine of db/db treated mice compared to non-treated mice ().
Table 1. AICAR improves kidney parameters in diabetic mice. Treatment of db/db mice with AICAR showed no significant changes in body weight, kidney weight and kidney weight/body weight compared to non-treated mice. On the other hand, significant decreased in hyperglycemia, proteinuria, albuminuria and urine creatinine were detected in AICAR treated mice compared to non-treated mice.
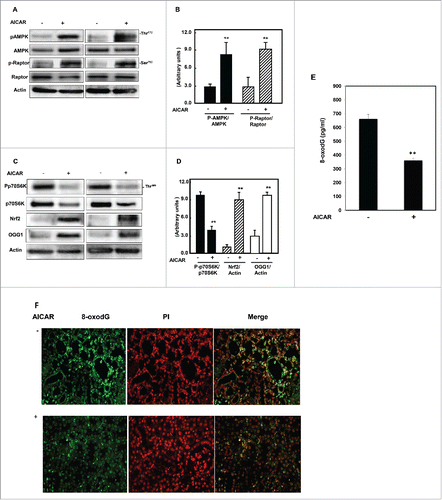
AICAR upregulates AMPK and inhibits mTORC1 to increase OGG1/Nrf2 and decrease DNA damage in diabetic mice
AICAR activates AMPK through its phosphorylation at Thr172 in response to changes in cellular ATP levels. Next, we tested the effect of AICAR treatment on regulation of AMPK/mTORC1 and OGG1/Nrf2 protein expression in diabetic kidney of db/db mice. Kidney cortex homogenates of treated and non-treated mice were analyzed by immunoblotting. Mice treated with AICAR showed a significant increase in AMPK phosphorylation at Thr172 compared to non-treated mice. AMPK activation associated with increase in phosphorylation (Ser792) and dissociation of raptor from mTORC1 to inhibit mTORC1 expression (). In addition, decrease in mTORC1 expression is associated with a significant increase in transcription factor Nrf2 and OGG1 protein expression in treated mice compared to non-treated mice (). Increase OGG1 protein expression in kidney of mice treated with AICAR resulted in significant decrease in accumulation of the oxidative DNA damage, 8-oxodG (pg/ml), quantitatively measured by 8-oxodG Stress MARQ kit () compared to non-treated animals (). These data was confirmed using fluorescent labeling method of immunostaining of 8-oxodG in kidney sections of non-treated and treated db/db mice (). These data suggest that AICAR present its effect through activation of AMPK and inhibition of mTORC1 through raptor, which leads to increased OGG1 protein expression by upregulation of Nrf2 and decreased the oxidative DNA damage (8-oxodG).
Figure 2. AICAR upregulates AMPK and inhibits mTORC1 to increase protein expression of Nrf2 and OGG1 and decrease 8-oxodG in kidney of diabetic mice. Immunoblot analysis shows that AICAR treatment increased phosphorylation of AMPK at Thr172 and resulted in significant decrease in mTORC1 measured by (A) increase in phosphorylation of raptor at Ser92 and (C) decrease in phosphorylation of p70S6k at Thr389 in kidney cortex of diabetic mice compared to non-treated mice. (C) Activation of AMPK resulted in significant increase in transcription factor Nrf2 and OGG1 protein expression in treated mice compared to non-treated mice. Actin was used as a loading control. (B&D) Histograms represent means ± SE (n = 4). (E) 8-OxodG levels were measured according to the instruction manual of 8-oxodG kits showed significant decrease in DNA damage in kidney of treated mice compared to non-treated mice. Significant difference from non-treated mice is indicated by **P < 0.01. Kidney sections of db/db mice (F) treated or non-treated with AICAR were stained with 8-oxodG antibody followed by FITC-anti-rabbit IgG as secondary antibodies. Staining of nucleus with propidium Iodide (PI) (red) and 8-oxodG with FITC (green) were detected using a filter with excitation range 450–490 nm and excitation at 535 nm.
AICAR activates OGG1 mediated AMPK activation and mTORC1 inhibition in renal proximal tubular cells exposed to HG
AMPK is an important upstream signaling intermediate intimately involved in the downregulation of the mTOR pathway. We tested whether AMPK activation can be restored by AICAR in cells treated with HG. Cells treated with HG for 48 h showed a significant decrease in AMPK phosphorylation at Thr172 that was associated with a significant decrease of raptor phosphorylation at Ser792 and increase p70S6K phosphorylation at Thr389 to activates mTORC1 (). Decrease in AMPK activity and increase in mTORC1 expression resulted in a significant decrease in Nrf2 and OGG1 protein expression (). Cells treated with 2 mM AICAR before being exposed to HG showed an increase in AMPK phosphorylation at Thr172 and raptor at Ser792 and a decrease in p70S6K phosphorylation (). Activation of AMPK and inhibition of mTORC1 by AICAR resulted in increased protein expression of Nrf2 and OGG1 (). In addition, cells treated with HG showed decrease in mRNA of OGG1, while cells pretreated with AICAR in presence of HG resulted in slight increase in mRNA of OGG1 (). On the other hand, cells grown in NG and treated with AICAR showed significant increase in mRNA OGG1 expression compared to non-treated cells. These data suggest that AICAR regulates OGG1 on the translation and transcription levels.
Figure 3. AICAR activates AMPK and decreases mTORC1 expression resulting in increased protein/mRNA expression and promoter activity of OGG1 and decreased 8-oxodG in HG-treated renal proximal tubular cells. High glucose significantly decreased AMPK phosphorylation and activates mTORC1 measured by decrease (A) in phosphorylation of raptor at Ser92 and (B) an increase in phosphorylation of p70S6k at Thr389 in MCT cells. (A) Cells treated with AICAR before exposure to HG show a significantly increased in activation of AMPK, which decreases mTORC1 expression. (B) Enhance AMPK activity and inhibition of mTORC1 by AICAR leads to upregulation of Nrf2 and OGG1 protein expression in cell exposed to HG. (C) RT-PCR of OGG1 was performed in RNA isolated from MCT cells grown in NG or HG and treated with AICAR. PCR products were analyzed on an ethidium bromide-stained gel. GAPDG was used a loading control. (D) AMPK activity was measured as phosphorylation levels of AMPK at Thr172 in HG or/and AICAR treated MCT cell lysates by AMPK ELISA kit. (E) A reporter plasmid containing the OGG1 promoter driving expression of the luciferase and a control Renilla reporter gene were co-transfected into the cells using LipofectAMINE Plus Reagent™. AICAR pretreatment reversed the inhibitory effect of HG on OGG1 promoter activity as well as significantly increased OGG1 activity in cells grown in NG. (F) DNA was extracted from treated and non-treated cells and digested with nuclease P1. The detection of dG and 8-oxodG was performed using 8-oxodG kits. Authentic standards of 8-oxodG were analyzed simultaneously. Experiment represent means±SE (n = 6). Significant difference from cells grown in normal glucose is indicated by *P < 0.01, cells grown in NG and treated with AICAR by **P < 0.01 and cells exposed to HG and treated with AICAR compared to cells grown in HG by #P < 0.01.
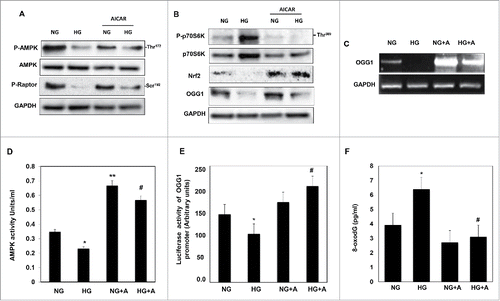
AMPK is a physiological cellular energy sensor that is activated by phosphorylation at Thr172 in response to changes in cellular ATP levels. MCT cells treated with HG showed significant decrease in AMPK activity measured as phosphorylation levels of AMPK at Thr172 using ELISA kit (). On the other hand, treatment the cells with AICAR under NG or HG condition showed significant increase in AMPK activity (). To test the effect of AICAR on transcriptional activity of OGG1, cells transfected with plasmid covering the region of Nrf2 that binds to OGG1 promoter. HG treated cells showed significant decrease in OGG1 promoter activity measured by luciferase assay () while treatment with AICAR resulted in increased the promoter activity of OGG1 in cells grown in NG or treated with HG (). Cells treated with 5 mM D-glucose and 20 mM L-glucose (Osmolality control) did not show any changes in regulation of P-AMPK, Nrf2 and OGG1 protein expression compared to cells grown in normal glucose (5 mM) (Fig. S1). Together, these data showed that AICAR significantly increased AMPK phosphorylation and subsequently increases protein and mRNA expression of OGG1 as well as promoter activity of OGG1 suggest that AICAR can function as an AMPK activator and mTORC1 inhibitor to activates OGG1 under HG exposure. To confirm the role of AICAR in reducing the oxidative DNA damage in cells treated with HG, DNA was isolated from non-treated and treated MCT cells. 8-OxodG levels measured by 8-oxodG kit showed significant accumulation of 8-oxodG amount in cells treated with HG while significant decrease in 8-oxodG levels were detected in cells treated with AICAR before exposed to HG () confirming role of AICAR in regulating DNA damage/repair pathway.
AMPK is a positive regulator of OGG1
Several approaches were demonstrated to test whether upstream or downstream signals of AMPK are involved in the regulation of DNA repair pathway. In the first approach, we have tested the effect of AMPK on the regulating the activity of OGG1 in cells exposed to HG condition. HEK293 cells transfected with DN-AMPK and treated with HG for 48h showed a significant decrease in Nrf2 and OGG1 protein expression compared to non-transfected cells grown under the same conditions (). In addition, transfected the cells with DN-AMPK showed significant decrease in OGG1 promoter activity in cells grown in NG or treated with HG (). These data suggest that AMPK is a major kinase involved in activation of DNA repair pathway.
Figure 4. Downregulation of AMPK resulted in decreased Nrf2 and OGG1 protein expression and led to a significant decrease in OGG1 promoter activity in renal proximal tubular cells treated with HG. (A) Cells transfected with DN-AMPK resulted in downregulation of AMPK in cells under both conditions of NG and HG. Decrease in AMPK activity resulted in significant downregulation of Nrf2 and OGG1 protein compared to cells transfected with control (nonspecific siRNA). (B) Cells co-transfected with DN-AMPK and a reporter plasmid construct carrying the OGG1 promoter showed a significant decrease in OGG1 promoter activity in cells grown under low and high concentrations of glucose indicating the role of AMPK as a major kinase regulate DNA repair activity. Experiment represent means ± SE (n = 6). Significant difference from cells grown in NG is indicated by *P < 0.01, cells grown in NG and transfected with DN-AMPK by **P < 0.01 and cells exposed to HG and transfected with DN-AMPK compared to cells grown in HG transfected with control by #P < 0.01. Downregulation of raptor resulted in decreased AMPK activity, Nrf2 and OGG1 protein expression as well as OGG1 promoter activity in renal proximal tubular cells treated with HG. (C) Cells transfected with siRNA against raptor showed a decrease of AMPK activity in cells under both conditions of NG and HG. Decrease of raptor expression resulted in significant downregulation of Nrf2 and OGG1 protein compared to cells transfected with control (nonspecific siRNA). (D) Cells co-transfected with siRNA against raptor and a reporter plasmid construct carrying the OGG1 promoter showed a significant decrease in OGG1 promoter activity in cells grown under low and high concentrations. Experiment represent means ± SE (n = 6). Significant difference from cells grown in NG is indicated by *P < 0.01, cells grown in NG and transfected with siRNA of raptor by **P < 0.01 and cells exposed to HG and transfected with siRNA of raptor compared to cells grown in HG transfected with control (nonspecific siRNA) by #P < 0.01. Downregulation of Akt resulted in increase Nrf2 and OGG1 protein expression and significant increase in OGG1 promoter activity in renal proximal tubular cells treated with HG. (E) Cells transfected with DN-Akt showed a decrease Akt phosphorylation at Ser473 in cells under both conditions of NG and HG. Decrease in Akt activity resulted in significant upregulation of Nrf2 and OGG1 protein compared to cells transfected with control (nonspecific siRNA). (F) Cells co-transfected with DN-Akt and reporter plasmid construct carrying the OGG1 promoter showed a significant increase in OGG1 promoter activity in cells grown under NG and HG indicating the role of Akt as a major kinase in the regulation of the DNA repair activity. Experiment represent means ± SE (n = 6). Significant difference from cells grown in NG is indicated by *P < 0.01, cells grown in NG and transfected with DN-Akt by **P < 0.01 and cells exposed to HG and transfected with DN-Akt compared to cells grown in HG transfected with control (nonspecific siRNA) by #P < 0.01. Downregulation of rictor resulted in increase Nrf2 and OGG1 protein expression as well as OGG1 promoter activity in renal proximal tubular cells treated with HG. (G) Cells transfected with siRNA against rictor showed a significant decrease in rictor expression resulted in upregulation of Nrf2 and OGG1 protein compared to cells transfected with control (nonspecific siRNA). (H) Cells co-transfected with siRNA against rictor and plasmid construct carried OGG1 promoter showed a significant increase in OGG1 promoter activity in cells grown under low and high concentrations. Experiment represent means ± SE (n = 6). Significant difference from cells grown in NG is indicated by *P < 0.01, and cells exposed to HG and transfected with siRNA of rictor compared to cells grown in HG transfected with control (nonspecific siRNA) by #P < 0.01.
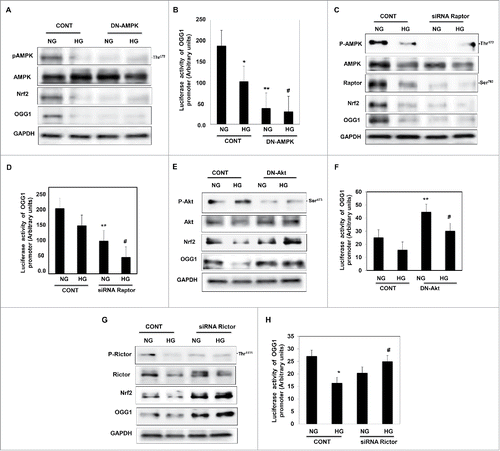
Raptor regulates OGG1 activity through AMPK
AMPK has inhibitory effects on mTOR through phosphorylation of raptor at Ser792 that causing decrease in mTORC1 expression. In the next experiment, we have demonstrated the effect of raptor on regulation of OGG1 as second approach. HEK 293 cells transfected with siRNA against raptor and treated with HG for 48h showed significant decrease in Nrf2 and OGG1 protein expression compared to cells transfected with control siRNA and grown under the same conditions (). In addition, transfected the cells with siRNA against raptor showed significant decrease in OGG1 promoter activity in cells grown in NG or treated with HG (). These data suggest that raptor is upstream target of OGG1 which upregulates Nrf2 or other transcription factors that are involved in the activation of DNA repair pathway.
Akt is a negative regulator of OGG1
Next we tested whether Akt/mTOR2 is a positive or negative regulator of DNA repair enzyme, OGG1 that may influence the level of oxidative DNA damage (8-oxodG). HEK293 cells transfected with DN-Akt and treated with HG for 48h. Cells transfected with DN-Akt showed significant increase in Nrf2 and OGG1 protein expression compared to non-transfected cells grown under the same conditions (). In addition, transfected cells with DN-Akt showed significant increase in OGG1 promoter activity in cells grown in NG or treated with HG (). These data suggest that Akt has a negative effect on the regulation of the DNA repair pathway.
Rictor regulates OGG1 activity through Akt
mTOR is a large protein kinase with 2 different complexes. One complex contains mTOR, GβL and raptor, which is a target of rapamycin. The other complex, insensitive to rapamycin, includes mTOR, GβL, Sin1, and rictor. The mTOR-rictor complex has a feedback to phosphorylate and activate Akt at Ser473. To test the effect of mTORC2 on regulation of OGG1, HEK293 cells transfected with siRNA against rictor and treated with HG for 48h showed a significant increase in Nrf2 and OGG1 protein expression compared to cells transfected with control siRNA and grown under the same conditions (). In addition, transfected cells with siRNA against rictor showed a significant increase in OGG1 promoter activity in cells grown in NG or treated with HG (). These data suggest that mTORC2 is negative regulator of OGG1 and support the role of mTORC1/2 as major kinases that are involved in regulating the DNA repair pathway.
Nrf2 is a major transcription factor for OGG1 activity
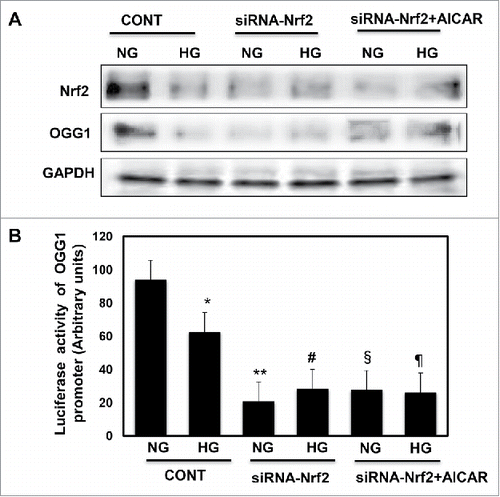
DNase footprinting assay showed that Nrf2 covers the region of the hOGG1 promoter from −35–21 (data not shown) and indicating that Nrf2 is one of important transcription factors that regulates OGG1 activity. In the third approach we tested the effect of regulation of Nrf2 in cells exposed to HG condition, HEK293 cells were transfected with siRNA against Nrf2 showed a significant decease in OGG1 protein expression (). In addition, cells transfected with siRNA of Nrf2 and treated with AICAR showed no increase in protein expression of Nrf2 and OGG1 compared to cells grown in NG. Promoter activity of OGG1 was significantly decreased in cells treated with HG while significant decrease was detected in cells transfected with siRNA of Nrf2, and cells transfected with siRNA and treated with AICAR OGG1 under both conditions of NG and HG (). These data confirmed that Nrf2 plays a critical role in regulating of OGG1 activity and indicates that Nrf2 involved in protecting renal cells from DNA damage during exposure to HG through upregulation the DNA repair pathway.
Figure 5. Downregulation of Nrf2 resulted in significant decrease in OGG1 protein expression and lead to sharp decrease in OGG1 promoter activity in renal roximal tubular cells treated with HG. (A) Cells transfected with siRNA against Nrf2 showed a significant decrease in OGG1 protein compared to cells transfected with control (nonspecific siRNA) and grown under both conditions of NG and HG. In addition, cells transfected with siRNA against Nrf2 and treated with AICAR showed no changes in protein expression of Nrf2 and OGG1. (B) Cells co-transfected with siRNA against Nrf2 and reporter plasmid construct carried OGG1 promoter showed a significant decrease in OGG1 promoter activity in cells grown under low and high concentrations. Cells transfected with siRNA against Nrf2 and treated with AICAR showed significant decrease in OGG1 promoter activity under both NG and HG conditions. Experiment represent means ± SE (n = 6). Significant difference from cells grown in NG compared to cells in HG is indicated by *P < 0.01, cells grown in NG and transfected with siRNA of Nrf2 by **P < 0.01, cells transfected with siRNA of Nrf2 and exposed to HG by #P < 0.01, cells grown in NG and transfected with siRNA of Nrf2+treated with AICAR by §P < 0.01, and cells transfected with siRNA of Nrf2 and exposed to HG+treated with AICAR by ¶P < 0.01.
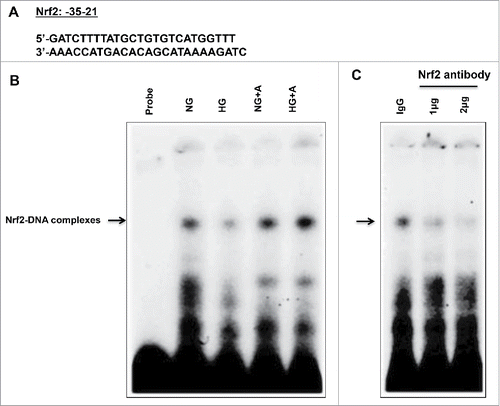
High glucose decreases the binding of Nrf2 to OGG1 promoter
The decrease in mRNA OGG1 expression is associated with decreased OGG1 promoter activity in cells exposed to HG (), suggesting decreased transcriptional mechanism. To further investigate the mechanism by which HG regulates OGG1 activity, EMSAs were performed to determine the capacity of Nrf2 to bind to the OGG1 promoter. Binding of Nrf2 to the OGG1 promoter region between −35−21 () was analyzed using nuclear extracts from MCT cells. Stronger protein binding to OGG1 promoter-specific DNA complexes was seen in nuclear extracts from cells grown in NG compared to cells exposed to HG for 48h (). On the other hands, an increased in protein binding of Nrf2 to OGG1 promoter-specific DNA complexes was detected in cells treated with AICAR and grown in HG compared to cells treated only with HG. To confirm the specificity of the protein-DNA interaction, the cell extracts were also pre-incubated with different concentrations (1-2ug) of an antibody recognizing Nrf2. A portion of the DNA-protein complexes was significantly decreased in the presence of the Nrf2 antibody, but not by IgG, indicating that Nrf2 is indeed a component of these complexes (). Taken together these data show that AICAR treatment improves the DNA repair function to reduce accumulation of oxidative DNA damage in renal cells in both cultured cells exposed to HG and diabetic mice. The action of AICAR was through upregulation of AMPK activity and downregulation of mTORC1 expression to decrease the loss of protein excreted in urine of treated diabetic mice.
Figure 6. High glucose treatment significantly reduces binding of Nrf2 to the OGG1 promoter element and AICAR treatment reversed the effect of HG in renal proximal tubular cells. (A) EMSA analysis of a DNA probe corresponding to the putative Nrf2 binding site in the OGG1 promoter. Labeled probes were incubated with nuclear extracts isolated from MCT cells grown in NG or HG and treated or non-treated with AICAR (2 mM). (B) Treatment of MCT cells with HG significantly reduced binding of Nrf2 to OGG1 promoter compared to cells grown in NG. While AICAR treatment showed significant increased in binding of Nrf2 to OGG1 promoter activity in cells grown in NG and cells exposed to HG. (C) The specificity of binding of the DNA/protein complex to Nrf2 was demonstrated by adding an Nf2 antibody (1 and 2ug) to the reaction mixture. Including the Nrf2 antibody in the reaction results in marked reduction of the specific DNA/protein complex.
Discussion
In this study, we provide first evidence that treatment diabetic mouse with AICAR upregulates the function of the DNA repair enzyme OGG1 and decreases accumulation of oxidative DNA damage (8-oxodG) to prevent renal damage. We also demonstrate that diabetes reduced Nrf2 protein expression and leads to downregulates OGG1 and enhancement of accumulation of oxidative DNA damage in proximal tubular cells in kidney cortex of db/db mice as well as in MCT cells treated with HG. In addition, our data indicate that treatment with AICAR improves kidney parameters of diabetic mice through decreased in proteinuria, albuminuria and urine creatinine in diabetic mice. Moreover, treatment with AICAR increased the AMPK activity, decreased mTORC1 expression and enhanced Nrf2 protein expression to upregulate OGG1 and decreased 8-oxodG levels in diabetic mice. Several approaches were demonstrated to investigate the mechanism by which AICAR regulates DNA damage/repair pathways in renal proximal tubular cells exposed to HG. Cells treated with AICAR before being exposed to HG showed significant increase in protein, mRNA and promoter activity of OGG1 as well as decreased 8-oxodG levels compared to cells treated with HG. Downregulation of AMPK and raptor decreased Nrf2 and OGG1 protein as well as the promoter activity of OGG1 suggesting that AMPK is a major kinase that activates DNA repair pathways. In addition, downregulation of Akt and rictor resulted in increase Nrf2 and OGG1 protein and promoter activity of OGG1 suggests that Akt/rictor is upstream signal that regulates OGG1. Moreover, decreased Nrf2 protein expression by siRNA resulted in significant downregulation of protein expression and promoter activity of OGG1 indicating that Nrf2 is a major transcription factor that activates OGG1. We also showed that HG decreased the binding of Nrf2 to the promoter of OGG1 while AICAR reversed these changes to improve OGG1 function and prevent accumulation of oxidative DNA damage in renal cells. Taken together, these results indicate that AICAR acts as an activator of AMPK and an inhibitor of mTOR pathway to regulate DNA damage/repair pathway and improve kidney parameters in diabetes.
AMPK is a physiological cellular energy sensor that is activated by phosphorylation at Thr172 in response to changes in cellular ATP levels. Previous studies show that AMPK is significantly decreased in diabetic animals as well as in renal cells exposed to HG.Citation18,19 On the other hand, pharmacological activation of AMPK such as metformin may be important for the prevention of obesity and associated metabolic diseases.Citation20 AMPK is thought to have 2 inhibitory effects on mTOR: (A) through upregulation of tuberin and promoting its Rheb-GAP activity, and (B) it regulates raptor through its phosphorylation at Ser792 causing decrease in mTORC1 expression. The regulatory associated protein of mTORC1 (raptor) was identified as an mTOR binding partner that mediates mTOR signaling to downstream targets. Inactivation of AMPK by HG influences the mTOR-raptor interaction. AMPK inhibits raptor through its phosphorylation at Ser792.Citation21 This phosphorylation is essential for inhibition of the raptor-containing mTOR complex 1 (mTORC1) and induces cell cycle arrest when cells are stressed for energy. Therefore, activation of AMPK by AICAR and inhibiting mTORC1 suggest that raptor is a critical switch in regulation of DNA repair pathway enzyme in diabetes. Our data confirm these observations and show that inactivation of AMPK represents a very proximal step in the intracellular signaling mTORC1 pathway triggered by HG to downregulates the DNA repair enzyme.
Pharmacologically targeting the gene-expression networks is attractive concept to prevent diabetes complications. Our data showed that AMPK activator, AICAR, blocks the effect of HG to activate of mTORC1 and subsequently downregulates DNA repair activity indicates that AMPK is a major kinase mediates activation of DNA repair pathways that prevent the harmful action of HG in renal cells. The concept that the AMPK/tuberin pathway regulates mTORC1 is supported by other observations that phosphorylation of raptor at Ser792 through AMPK causes decrease in mTORC1 expression.Citation21 On the other hand, rictor is phosphorylated at Thr1135 by p70S6K, which negatively regulates mTORC2 protein complex as part of a negative feedback mechanism controlling Akt activity.Citation22 Therefore, our data showed 2 opposite functions of mTOR, mTORC1 as a positive regulator and mTORC2 as a negative regulator of the DNA repair enzyme.
The fact that the exposure of cells to HG is sufficient to elicit oxidative stress on proximal tubule epithelial cells suggests that episodes of increases in glucose may contribute to cell injury and to epithelial cell dysfunction.Citation23 Recently, several studies have indicated the preventive effects of Nrf2 on HG-induced oxidative damage in the cultured cells and potentially on the diabetic complications in animal models.Citation24 We showed previously that HG–induced tuberin phosphorylation by Akt is associated with biological consequences, namely a decrease in OGG1 expression and increased 8-oxodG levels.Citation25 Importantly, 8-oxodG appears to play a role in tissue cell injury via the induction of apoptotic cell death.Citation26 Increased number of 8-oxodG–positive islet cells was found in the human pancreas from type 2 diabetic subjects.Citation27 Our observation in renal proximal cells was confirmed in vivo where we found that AICAR activates AMPK at Thr172 resulting inactivation of mTROC1 through to increase Nrf2 in diabetic mice.. In addition, the increased binding of Nrf2 to OGG1 promoter resulted in an increase of DNA repair function and decrease accumulation of oxidative DNA damage in renal cells exposed to HG as well as in diabetic mice treated with AICAR. The increased in AMPK activity by AICAR treatment did not correlated with increase in OGG1 promoter activity under normal glucose condition while much increase in OGG1 promoter activity under high glucose condition+AICAR suggesting that OGG1 promoter response is more effective when the cells expose to oxidative stress. These data provided evidence of role of AICAR in improving OGG1 promoter activity under high glucose condition and prevent accumulation of oxidative DNA damage. The decrease in protein expression of OGG1 and Nrf2 didn't associate identically with OGG1 promoter activity since the promoter assay measured the OGG1 function, which not exactly reflected on the protein expression of OGG1.
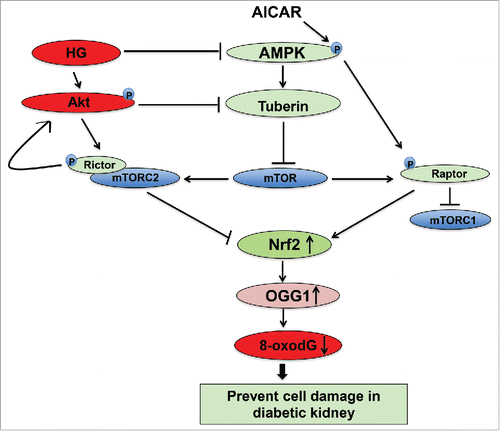
In summary, these data describe a novel role of AICAR in preventing diabetic renal damage through modulation of the AMPK/mTOR pathway to activate DNA repair function and reduce accumulation of oxidative DNA damage in diabetes. AICAR activates AMPK and leads to inhibition of mTOR. In addition, inactivation of AMPK by DN-AMPK and mTORC1 by siRNA against raptor resulted in decreased the promoter activity and protein expression of OGG1 through downregulation of Nrf2. On the other hand, inactivation of Akt by DN-Akt and mTORC2 by siRNA against rictor resulted in increased the promoter activity of OGG1 through upregulation of Nrf2. These data suggest that AICAR activates AMPK and inhibits binding of raptor to mTORC1 to increase OGG1 activity. Inactivation of AMPK results in downregulation Nrf2 and leads to decrease the functional activity of OGG1 and increase DNA damage, 8-oxodG ). On the other hand, inactivation of Akt and inhibition of mTORC2 results in upregulation of OGG1 activity to reduce accumulation of oxidative DNA damage in renal cells. The consequences steps of upregulation and downregulation of major signals that activate the DNA repair pathways and prevent cell damage lead to improved kidney parameters in diabetic patients under the therapeutic effect of AMPK activator. Collectively, these data provide a novel mechanism by which AICAR improve the kidney parameters and prevents the progression of renal diabetic complications.
Figure 7. Proposed model for the role of AICAR in preventing kidney damage in diabetes. AICAR activates AMPK and inhibits binding of raptor to mTORC1 to decrease mTOR and increase OGG1 activity. Inactivation of AMPK results in downregulation Nrf2 and leads to decrease the functional activity of OGG1. On the other hand, inactivation of Akt and inhibition of mTORC2 results in upregulation of OGG1 activity to reduce accumulation of oxidative DNA damage in renal cells. The consequences steps of upregulation and downregulation of major signals that activate the DNA repair pathways and prevent cell damage lead to improved kidney parameters in diabetic patients under the therapeutic effect of AMPK activator.
Materials and methods
Cell culture
The murine proximal tubular epithelial (MCT) cells were grown in DMEM containing 10% fetal bovine serum, 5-mmol/l glucose, 100-units/ml penicillin, 100μg/ml streptomycin, and 2mmol/l glutamine. Confluent cells were growth-arrested overnight in serum-free DMEM before experiments.
AICAR treatment
MCT cells were grown to 80–90% confluency in 60 mm petri dish in normal glucose (NG) (5 mM) or Hg (25 mM). Cells were treated with AICAR (2 mM) for 48 h before exposed to HG. AICAR was obtained from Cayman Chemical (Ann Arbor, MI). The cells were lysed in laysis buffer as described previously.Citation28 Cell lysates were used for Western blot analysis.
AMPK activity assay
AMPK activity was measured in MCT cell lysates using AMPK ELISA Kit. A monoclonal capture antibody specific for AMPKα coated onto the wells of the 96-well plate was provided by Invitrogen kit. Standard of AMPK [p-T172] was used to calculate the activity of AMPK in the unknown samples. The AMPK activity (Units/ml) was measured at 450nm using microplate reader.
Transcriptional activity of OGG1 promoter
A luciferase reporter plasmid containing the OGG1 promoter (courtesy of Dr. P. Radicella) was used to determine OGG1 gene transcription activity.Citation29 Human Embryonic Kidney 293 (HEK 293) cells were grown in 12-well plates to 60–70% confluence, then co-transfected with both renilla and OGG1 plasmid covering the region of Nrf2, −35–21, that binds to OGG1 promoter using Oligofectamine and Plus Reagent as described previously.Citation30 Cells were treated with HG (25 mM) and/or AICAR (2 mM) 48 hours before harvesting the cells for luciferase assay. In addition, DN-AMPK, DN-Akt, siRNA raptor or siRNA rictor, siRNA Nrf2 was transfected into the cells 48 h before treatment with HG. Luciferase activity of OGG1 was determined using the Luciferase Reporter Assay System in a luminometer (Promega, WI) and normalized to Renilla luciferase.
Downregulation of AMPK and Akt
HEK 293 cells grown to 60–70% confluency in complete medium in 6-well plates were transfected with a recombinant plasmid expressing DN-AMPK. The plasmid containing AMPK carrying K45R mutation of the α1-subunit (pCAGGS) or plasmid expressing DN-Akt was transfected into the cells using lipofectamine and Lipo-plus reagent (Invitrogen) as described previously.Citation30 Cells were treated with HG (25 mM) 48h before harvesting for Western blot analysis.
Downregulation of raptor, rictor and Nrf2 by siRNA
HEK 293 cells were grown in 6-well plates in NG medium. Selected siRNA duplexes against raptor or rictor or Nrf2 and control siRNA were obtained as a kit from Santa Cruz Biotechnology (Santa Cruz, CA). Cells were infected with specific siRNA or nonspecific siRNA duplexes (control) as described previously.Citation31 Cells were treated with HG for 48 hours before harvesting for Western blot analysis.
Protein extraction and immunoblot analysis
Protein concentration of the cell lysates or kidney cortex homogenates was determined with the Bradford reagent using bovine serum albumin as a standard.Citation32 Western blot analysis was performed as previously described.Citation33 P-p70S6K, p70S6K P-AMPK, AMPK, p-Akt, Akt, p-raptor, raptor, p-rictor, and rictor antibodies were purchased from Cell Signaling Technology. Mouse ß-actin antibody was purchased from Oncogene Research Products and Nrf2 antibody from Abcam (Cambridge, MA). OGG1 and GADPH antibodies were obtained from Santa Cruz Biotechnology. Expression of each protein was quantified by densitometry using NIH Image 1.62 software.
mRNA analysis by RT-PCR analysis
RNA was extracted from MCT cells using RNA Isolation Solvent (Tel-Test). RNA was quantified by spectrophotometer at 260 nm, and its integrity was tested by formaldehyde/agarose gel electrophoresis. RTPCR was performed as previously describedCitation28 using the primers the primers (5′-3-AACATTGCTCGCATCACTGGC/5′-GATGTCCACAGGCACAGCCTG) for OGG1. The amplified product was 356-bp long. For GAPDH, an internal control of amplification, upstream/reverse primers were 5′- GCCACCCAGAAGACTGTGGAT /5′- GAAGGCCATGCCAGTGAGCT synthesizing a 528-bp product. The PCR products were analyzed by electrophoresis on agarose gels and ethidium bromide staining.
Electrophoretic mobility shift assay of Nrf2
Nuclear proteins were extracted from MCT cells as described previously.Citation28 The protein concentration of the nuclear extracts was determined using Bradford method.Citation31 Electrophoretic mobility shift assay (EMSA)-binding reactions were incubated in a 20 μl final volume for 20 min at room temperature containing 5 μg of the nuclear extract, 20–30 fmol of the 5′ end-labeled double-stranded 25 bp oligonucleotide: 5′-GATCTTTTATGCTGTGTCATGGTTT-3′, covering the region of the Nrf2 that binds to OGG1 promoter, and 1 μl of poly (dI-dC). The super shift assays were performed by pre-incubating nuclear extracts with 1 or 2 μg of Nrf2 antibody (Abcam, Cambridge, MA) into the reaction. The reaction was carried out at room temperature for 30 min prior to adding the radiolabelled probe. The complexes were resolved using a 5% non-denaturing polyacrylamide gel. The gels were dried and exposed overnight at −70°C.
Animals
Male db/db mice around 4 weeks old (Strain BKC.Cg.m +/+Leprdb/J) were purchased from Jackson Laboratory. The animals were allowed food and water ad libitum prior to and during the experiments. The mice were divided into 2 groups. Mice in group 1 (controls) were injected with an equal amount of DMSO. Mice in group 2 were injected i.p. with 2 mg/kg body weight AICAR in DMSO 5 d/week for 4 weeks. Injections were carried out under isofluorane inhalation anesthesia (Abbott, Abbott Park, IL). Animals were euthanized at 4 weeks and the kidneys were removed rapidly for dissection and biochemical analysis. The study has been approved by the Institutional Review Board of The University of Texas Health Science Center at San Antonio, TX.
Homogenates of kidney cortex were prepared as described previously.Citation33 Urine protein concentration was measured in 24-collected urine in each animal before after treatment with AICAR. Albumin was measured in 24 urine collection of each animal by ELISA ALPCO kit (Salem, NH).Citation34 Urinary creatinine was measured in 2 µl urine samples using a kit purchased from Quidel (San Diego, CA, USA). Final absorbance was read at 450 nm in a Fusion Packard plate reader.
Immunostaining of 8-oxodG
A fluorescent labeling method of 8-oxodG immunostaining was performed in kidney sections of db/db mice as described previously.Citation35 FITC green signals for 8-oxodG were detected using a filter with excitation range 450–490 nm and propidium iodide (PI) red signals for nuclear DNA using a filter with excitation at 535 nm. Kidney sections were viewed and photographed using a Nikon Research microscope equipped for epifluorescence with excitation and band pass filters. To demonstrate staining specificity, control kidney sections were stained without primary antibody.
8-OxodG quantitation
DNA was isolated from MCT cells or frozen samples of kidney as described previously.Citation35 DNA purity and concentration was determined with spectrophotometer. Digestion of 8-oxodG was performed as described previously.Citation25 Authentic standards of 8-oxodG were analyzed along with every according to the instruction manual of 8-oxodG kit from Stress MARQ (Bioscience INC, Victoria, Canada).
Statistics
Data are presented as mean ± standard error. Statistical differences were determined using ANOVA followed by Student Dunnett's (Exp. versus Control) test using one trial analysis. P-values less than 0.01 and 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KCCY_S_1231259.pdf
Download PDF (65.1 KB)Funding
This work was supported in part by grants from the American Heart Association and Merit Review Award from South Texas Veterans Healthcare System (to S.L.H.).
References
- Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care 2002; 5:561-8; PMID:12172481; http://dx.doi.org/10.1097/00075197-200209000-00016
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414:813-20; PMID:11742414; http://dx.doi.org/10.1038/414813a
- Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol 2003; 14:S241-5; PMID:12874439; http://dx.doi.org/10.1097/01.ASN.0000077410.66390.0F
- Kasai H, Nishimura S. Formation of 8-hydroxyguanine by oxidative DNA damage, its repair and its mutagenic effects. In de Obe G. (ed.) Advances in mutagenesis Research. Springer-Verlag, Berlin, Germany, 1993; Vol. 4, pp.31-45.
- Nishikawa T, Sasahara T, Kiritoshi S. Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Diabetes Care 2003; 26:1507-12; PMID:12716813; http://dx.doi.org/10.2337/diacare.26.5.1507
- Inokio Y, Suzuki S, Hirai M, Suzuki C, Suzuki M, Toyota T. Urinary excretion of 8-oxo-7, 8-dihydro-2′-deoxyguanosine as a predictor of the development of diabetic nephropathy. Diabetologia 2002; 45:877-82; PMID:12107732; http://dx.doi.org/10.1007/s00125-002-0831-8
- Smart DJ, Chipman JK, Hodges NJ. Activity of OGG1 variants in the repair of prooxidant-induced 8-oxo-2′-deoxyguanosine. DNA Rep 2006; 5:1337-45; PMID:16861056; http://dx.doi.org/10.1016/j.dnarep.2006.06.001
- Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res 567:1-61; PMID:15341901; http://dx.doi.org/10.1016/j.mrrev.2003.11.001
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 1997; 236:313-22; PMID:9240432; http://dx.doi.org/10.1006/bbrc.1997.6943
- Jain AK, Bloom DA, Jaiswal AK. Nuclear import and export signals in control of Nrf2. J Biol Chem 2005; 280:29158-68; PMID:15901726; http://dx.doi.org/10.1074/jbc.M502083200
- Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5′-AMP-Activated Protein Kinase (AMPK) Is Induced by Low-Oxygen and Glucose Deprivation Conditions Found in Solid-Tumor Microenvironments. Mol Cell Biol 2006; 26:5336-47; PMID:16809770; http://dx.doi.org/10.1128/MCB.00166-06
- Guo D, Hildebrandt IJ, Prins RM, Soto H, Mazzotta MM, Dang J, Czernin J, Shyy JY, Watson AD, Phelps M, et al. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci U S A 2009; 106:12932-7; PMID:19625624; http://dx.doi.org/10.1073/pnas.0906606106
- Winder WW, Thomson DM. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem Biophys 2007; 47:332-47; PMID:17652779; http://dx.doi.org/10.1007/s12013-007-0008-7
- Boon H, Bosselaar M, Praet SF, Blaak EE, Saris WH, Wagenmakers AJ, McGee SL, Tack CJ, Smits P, Hargreaves M, et al. Intravenous AICAR administration reduces hepatic glucose output and inhibits whole body lipolysis in type 2 diabetic patients. Diabetologia 2008; 51:1893-900; PMID:18709353; http://dx.doi.org/10.1007/s00125-008-1108-7
- Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol 2005; 25:9554-75; PMID:16227605; http://dx.doi.org/10.1128/MCB.25.21.9554-9575.2005
- Dong Y, Zhang M, Wang S, Liang B, Zhao Z, Liu C, Wu M, Choi HC, Lyons TJ, Zou MH. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes 2010; 59:1386-96; PMID:20299472; http://dx.doi.org/10.2337/db09-1637
- Winder WW. Can patients with type 2 diabetes be treated with 5′-AMP-activated protein kinase activators? Diabetologia 2008; 51:1761-4; PMID:18696044; http://dx.doi.org/10.1007/s00125-008-1115-8
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005; 54:1615-25; PMID:15919781; http://dx.doi.org/10.2337/diabetes.54.6.1615
- Halseth AE, Ensor NJ, White TA, Ross SA, Gulve EA. Acute and chronic treatment of ob/ob and db/db mice with AICAR decreases blood glucose concentrations. Biochem Biophys Res Commun 2002; 294:798-805; PMID:12061777; http://dx.doi.org/10.1016/S0006-291X(02)00557-0
- Caton PW, Kieswich J, Yaqoob MM, Holness MJ, Sugden MC. Metformin opposes impaired AMPK and SIRT1 function and deleterious changes in core clock protein expression in white adipose tissue of genetically-obese db/db mice. Diabetes Obes Metab 2011; 13:1097-104; PMID:21733059; http://dx.doi.org/10.1111/j.1463-1326.2011.01466.x
- Lee MN, Ha SH, Kim J, Koh A, Lee CS, Kim JH, Jeon H, Kim DH, Suh PG, Ryu SH. Glycolytic flux signals to mTOR through glyceraldehyde-3-phosphate dehydrogenase-mediated regulation of Rheb. Mol Cell Biol 2009; 29:3991-4001; PMID:19451232; http://dx.doi.org/10.1128/MCB.00165-09
- Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol Cell Biol 2010; 30:908-21; PMID:19995915; http://dx.doi.org/10.1128/MCB.00601-09
- Samikkannu T, Thomas JJ, Bhat GJ, Wittman V, Thekkumkara TJ. Acute effect of high glucose on long-term cell growth: a role for transient glucose increase in proximal tubule cell injury. Am J Physiol Renal Physiol 2006; 291:F162-75; PMID:16467130; http://dx.doi.org/10.1152/ajprenal.00189.2005
- Cui W, Li B, Bai Y, Miao X, Chen Q, Sun W, Tan Y, Luo P, Zhang C, Zheng S, et al. Potential role for Nrf2 activation in the therapeutic effect of MG132 on diabetic nephropathy in OVE26 diabetic mice. Am J Physiol Endocrinol Metab 2013; 304:E87-99; PMID:23132297; http://dx.doi.org/10.1152/ajpendo.00430.2012
- Simone S, Gorin Y, Velagapudi C, Habib SL. Mechanism of oxidative DNA damage in diabetes: tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2′-deoxyguanosine-DNA glycosylase. Diabetes 2008; 57:2626-36; PMID:18599524; http://dx.doi.org/10.2337/db07-1579
- Roseborough G, Gao D, Chen L, Trush MA, Zhou S, Williams GM, Wei C. The mitochondrial K-ATP channel opener, diazoxide, prevents ischemia-reperfusion injury in the rabbit spinal cord. Am J Pathol 2006; 168:1443-51; PMID:16651612; http://dx.doi.org/10.2353/ajpath.2006.050569
- Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese type II diabetic patients. Diabetologia 2002; 45:85-96; PMID:11845227; http://dx.doi.org/10.1007/s125-002-8248-z
- Habib SL, Riley DJ, Bhandari B, et al. Tuberin Regulates the DNA Repair Enzyme OGG1. Am J Physiol Renal Physiol 2008; 294:F281-90; PMID:17989114; http://dx.doi.org/10.1152/ajprenal.00370.2007
- Dhenaut A, Boiteux S, Radicella JP. Characterization of the hOGG1 promoter and its expression during the cell cycle. Mutat Res 2000; 461:109-18; PMID:11018584; http://dx.doi.org/10.1016/S0921-8777(00)00042-2
- Habib SL, Kasinath BS, Arya RR, Vexler S, Velagapudi C. Novel mechanism of reducing tumourigenesis: upregulation of the DNA repair enzyme OGG1 by rapamycin-mediated AMPK activation and mTOR inhibition. Eur J Cancer 2010; 46:2806-20; PMID:20656472; http://dx.doi.org/10.1016/j.ejca.2010.06.117
- Habib SL, Bhandari BK, Sadek N, Abboud-Werner SL, Abboud HE. Novel Mechanism of Regulation of the DNA repair enzyme OGG1 in Tuberin-deficient Cells. Carcinogenesis 2010; 31:2022-30; PMID:20837600; http://dx.doi.org/10.1093/carcin/bgq189
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248-54; PMID:942051; http://dx.doi.org/10.1016/0003-2697(76)90527-3
- Habib SL, Simone S, Barnes JJ, Abboud HE. Tuberin Haploinsufficiency is Associated with the Loss of OGG1 in Rat Kidney Tumors. Mol Cancer 2008; 7:10-14; PMID:18218111; http://dx.doi.org/10.1186/1476-4598-7-10
- Habib SL, Yadav M, Tizani S, Bhandari B, Valente AJ. Tuberin inhibits production of the matrix protein fibronectin in diabetes. J Am Soc Nephrol 2012; 2310:1652-62; http://dx.doi.org/10.1681/ASN.2012030285
- Habib SL, Phan MN, Patel SK, Li D, Monks TJ, Lau SS. Reduced constitutive 8-oxoguanine-DNA glycosylase expression and impaired induction following oxidative DNA damage in the tuberin deficient Eker rat. Carcinogenesis 2003; 24:573-82; PMID:12663520; http://dx.doi.org/10.1093/carcin/24.3.573
