The serum/glucocorticoid-regulated kinase (SGK) family is formed by 3 different isoforms (SGK1-3) and belongs to the AGC kinase group of the human kinome. SGK1 was initially discovered in a differential screening of transcripts in response to corticosteroid treatment.Citation1 Further studies showed that SGK kinases are rapidly induced upon treatment with several steroid hormones and growth factors, likely due to the presence of hormone responsive elements in the promoter region of the gene. Given that both mRNA and protein have a short half-life, the increase in SGK1 is transient.
The role of SGK has been widely studied in the context of epithelial ion transport, playing key roles in Na+ reabsorption and renal K+ secretion.Citation2 However, the importance of the SGK kinase family in the field of cancer research has been overlooked for several years.
Activation of PI3K by somatic mutations in PIK3CA, the gene encoding for PI3Kα, or amplification of upstream mitogenic receptors such as HER2, leads to increased phosphatidylinositol-(3,4,5)-triphosphate (PIP3) synthesis at the plasma membrane. This lipid can be recognized by several protein effectors that contain a pleckstrin homology domain (PH). Among these, PDK1 and AKT kinases have the ability to interact with PIP3 at the plasma membrane, where PDK1 and mTORC2 will sequentially phosphorylate and activate AKT. In the case of SGK's, the mechanism of upstream activation is very similar: phosphorylation at the hydrophobic motif is carried out by mTORC2, where PDK1 will subsequently dock to phosphorylate the activation loop.Citation3 Stimulation of the PI3K/AKT pathway will have several cellular effects that include but are not limited to cell growth, proliferation, increased metabolism, and apoptosis evasion. As a result, pharmacologic inhibition of this oncogenic pathway is of great interest in the field of cancer research.
Vasudevan and colleagues were the first to link SGK3 with breast cancer by finding this kinase in a targeted shRNA screening aimed to uncover PDK1 substrates involved in AKT-independent growth.Citation4 While SGK kinases are not directly implicated in oncogenic transformation, they seem to play a role in cell survival under circumstances in which AKT activity has been challenged. The reason for this peculiarity probably resides in the fact that the kinase domains of AKT and SGK's are highly homologous, leading to a similar subset of substrates that can be phosphorylated at the shared consensus motif RXRXX(S/T). This suggests that both kinases could substitute for each other. In this context, the abundance of these kinases or their binding affinities to the substrates could determine the predominant substrate that will be phosphorylated. Taking this into consideration, it is reasonable to speculate that SGK kinases are related to resistance to AKT inhibition. Indeed, the laboratory of Dario Alessi has previously reported that high levels of SGK1 in breast cancer cell lines predict intrinsic resistance to these inhibitors in vitro.Citation5
In a study recently published in Cancer Cell,Citation6 we have found that SGK1 levels are also associated with resistance to PI3Kα inhibitors, currently under clinical development. This observation is intriguing because although SGK1 lacks the pleckstrin homology (PH) domain found in AKT, evidence suggests that the activity of this kinase is tightly regulated by PI3K. In resistant cells, inhibition of PI3Kα leads to complete inhibition of AKT activity, as a result of decreased PIP3 levels in the plasma membrane. However, SGK1 remains partially active in the presence of PI3Kα inhibitors, which could be explained by either residual mTORC2 activity or due to the presence of a PIP3-independent pool of mTORC2.
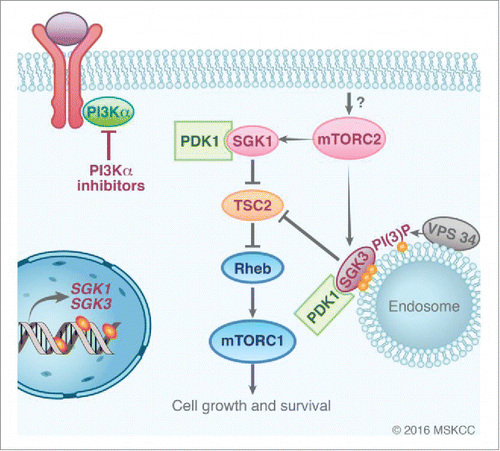
In this work, we have also described that pharmacologic inhibition of PDK1 is effective in breast cancer cells that are intrinsically resistant to PI3Kα inhibitors and overexpress SGK1. Because PDK1 is responsible for the phosphorylation of SGK's at the activation loop, concomitant inhibition of PDK1 and PI3K is expected to abrogate SGK1 and AKT activity, respectively. We have also shown that SGK1 has the ability to phosphorylate TSC2, a component of the TSC complex that negatively regulates mTORC1, leading to the activation of mTORC1 (). This also seems to be the case for SGK3, which was recently shown to be transcriptionally upregulated upon long-term exposure to either PI3K or AKT inhibitors in cells sensitive to these agents.Citation7 When upregulated, SGK3 re-activates the mTORC1 pathway by the same mechanism, although the case of SGK3 is unique as it requires the presence of phosphatidylinositol 3-phosphate (PI(3)P) in the endosomal membranes. This would suggest that inhibition of VPS34, the class III PI3K responsible for synthesising PI(3)P, may enhance the activity of PI3K/AKT inhibitors. In both studies, characterization of compounds that target either SGK1 or SGK3 have potent antitumor effects when combined with PI3KαCitation6 or AKT inhibitors,Citation7 providing further rationale for the inhibition of these kinases in cancer patients.
Figure 1. Sustained mTORC1 activity in the absence of PI3K/AKT signaling by SGK kinases. SGK1/3 are transcriptionally regulated and activated by mTORC2 and PDK1 phosphorylation. SGK3 is recruited at the endosomal membrane interacting with PI(3)P, which results from VPS34 enzymatic activity. SGK kinases have the ability to phosphorylate TSC2, the GTPase Activating Protein for RHEB, the upstream activator of mTORC1.
Future work will be required to understand the mechanism by which mTORC2 is activated, discover novel substrates of SGK kinases, and develop highly specific and potent SGK inhibitors that could be tested in the clinic.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol 1993; 13:2031-40; PMID:8455596; http://dx.doi.org/10.1128/MCB.13.4.2031
- Loffing J, Flores SY, Staub O. SGK kinases and their role in epithelial transport. Annu Rev Physiol 2006; 68:461-90; PMID:16460280; http://dx.doi.org/10.1146/annurev.physiol.68.040104.131654
- Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J 2008; 416:375-85; PMID:18925875; http://dx.doi.org/10.1042/BJ20081668
- Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, Hennessy BT, Tseng H, Pochanard P, Kim SY, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell 2009; 16:21-32; PMID:19573809; http://dx.doi.org/10.1016/j.ccr.2009.04.012
- Sommer EM, Dry H, Cross D, Guichard S, Davies BR, Alessi DR. Elevated SGK1 predicts resistance of breast cancer cells to Akt inhibitors. Biochem J 2013; 452:499-508; PMID:23581296; http://dx.doi.org/10.1042/BJ20130342
- Castel P, Ellis H, Bago R, Toska E, Razavi P, Carmona FJ, Kannan S, Verma CS, Dickler M, Chandarlapaty S, Brogi E, et al. PDK1-SGK1 Signaling Sustains AKT-Independent mTORC1 Activation and Confers Resistance to PI3Kα Inhibition. Cancer Cell 2016; 30:229-42; PMID:27451907; http://dx.doi.org/10.1016/j.ccell.2016.06.004
- Bago R, Sommer E, Castel P, Crafter C, Bailey FP, Shpiro N, Baselga J, Cross D, Eyers PA, Alessi DR. The hVps34-SGK3 pathway alleviates sustained PI3K/Akt inhibition by stimulating mTORC1 and tumour growth. EMBO J 2016; 35(17):1902-22; PMID: 27481935; http://dx.doi.org/10.15252/embj.201693929
