Life expectancy in the developed countries is continuously increasing. However, age-related diseases lead to late life complications and remain the most prevalent cause of mortality. One of the cellular components that is present in sites of age-related pathologies and accumulates during aging is senescent cells.Citation1 These cells are formed when a stress signal triggers terminal cell cycle arrest in proliferating cells. Entrance to a state of senescence deprives damaged cells of their proliferative potential and thus limits tumorigenesis and tissue damage.Citation1 Despite the protective role of cellular senescence, the long term presence of senescent cells is harmful to their environment. These cells secrete a plethora of pro-inflammatory factors that might aid their removal by the immune system.Citation1-3 However, at advanced age senescent cells gradually accumulate in tissues and the secretory phenotype promotes a chronic “sterile” inflammation which is a hallmark of unhealthy aging. Elimination of senescent cells in mice by a genetic approach led to a decreased burden of age-related disorders, and an increased median survival of the mice. Therefore, pharmacological elimination of senescent cells in-vivo is a promising strategy for treatment of age-related diseases associated with accumulation of senescent cells. An attractive method to implement this strategy would be to induce apoptosis preferentially in senescent cells. The scientific basis of this approach relies on an understanding of the molecular mechanisms that distinguish the regulation of apoptosis in senescent cells from other cells.
Resistance of senescent cells to both extrinsic and intrinsic pro-apoptotic stimuli testifies for complex regulation of apoptosis in these cells. We recently demonstrated that senescent cells, induced to senesce by different kind of insults, upregulate proteins of the anti-apoptotic BCL-2 family.Citation4 Combined knockdown of these proteins or their inhibition by a small molecule inhibitor, ABT-737, selectively skew cell-fate decision in senescent epithelial cells in-vivo toward apoptosis. Therefore, the expression of BCL-2 family members endowed senescent cells with resistance to apoptosis. The senolytic activity of ABT-737 molcule was demonstrated in 2 independent in-vivo models of senescence. In the first model, DNA damage-induced senescent cells were formed in the lungs upon ionizing irradiation of mice. Administration of ABT-737 rapidly reduced the number of senescent cells, concomitantly with an increase in apoptosis. In the second model, activation of p14ARF in the skin epidermis of K5-rtTA/tet-p14 mice resulted in senescent cell formation, in a p53-dependent manner. Administration of ABT-737 to these mice following induction of p14ARF was able to dramatically reduce the number of senescent cells. In a parallel study, administration of ABT-263, a paralog inhibitor of ABT-737, showed clearance of senescent cells in the haematopoietic system.Citation5 Therefore, BCL-2 family inhibitors preferentially induce apoptosis in senescent cells in variety of in-vivo systems.
Alongside with the BCL-2 family inhibitors, other approaches for selective elimination of senescent cells, also termed senolytic approaches, have been identified. For example, the combination of 2 drugs, Dasatinib and Quercetin, was shown to exert killing potential of senescent preadipocyte and endothelial cells.Citation6 Elimination of senescent cells could also be achieved by adapting tools from the field of cancer therapy. One such possibility is utilization of common immunotherapy practices following identification of senescence-specific markers. The immune system is a natural resource that is able to recognize and eliminate senescent cells.Citation2 Using its properties in combination with immunotherapy approaches or with emerging senolytic drugs might lead to more specific and efficient elimination of senescent cells. However, no matter what would be the approach of choice, it is necessary to keep in mind that senescent cells participate in variety of essential physiological functions such as in wound healing, tumor suppression, regulation of glucose levels and embryonic development. In order to develop efficient senolytic approaches it is necessary to dissect beneficial and detrimental functions of senescent cells in different physiological and pathophysiological conditions using in-vivo models.
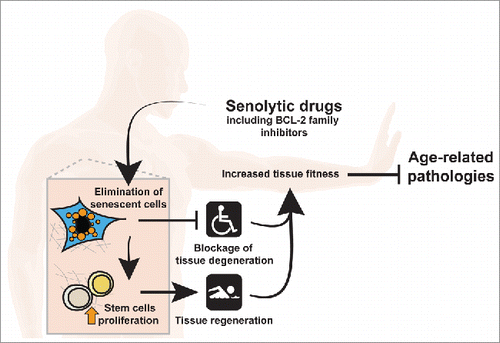
Successful development of senolytic drugs will bring senescent cells to the forefront of anti-aging therapies. However, it is necessary to understand the effect of elimination of senescent cells on diverse cell communications in the complex tissues. Elimination of senescent cells by ABT-737 or ABT-263 was followed by increased proliferation of stem cells in both skin and haematopoietic system.Citation4,5 These results suggest that senolytics can have an impact on tissue regeneration and can potentially be used in regenerative medicine. This approach will combine elimination of damaged cells with stimulation of proliferation of healthy progenitors, in a way that could restore tissue fitness in diseases associated with reduced tissue function (). In addition to such diseases, age is also a significant risk factor for the development of several types of cancer. Senescent stromal cells can establish a tumor supporting microenvironment, including local immunosuppression that drives tumorigenesis and metastasis, suggesting that pharmacological elimination of senescent cells can halt this process.Citation7 Taken together, new senolytic approaches are being discovered. They have an exciting potential to extend human healthspan, but need to be taken with caution in order to enable senescent cells to perform their physiological function. Understanding the mechanism of action under specific conditions in-vivo will direct to efficient and safe usage of senolytic drugs in the future.
Figure 1. Senolytic drugs can become a future regenerative medicine. Treatment with senolytic drugs results in the elimination of senescent cells, thus blocking tissue degeneration and late life complications. In turn, elimination of senescent cells leads to the proliferation of stem cells, allowing tissue regeneration. This joined effect of senolytic drugs will restore tissue fitness and will help restraining age-related pathologies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 2015; 21:1424-35; PMID:26646499; http://dx.doi.org/10.1038/nm.4000
- Sagiv A, Burton DGA, Noshayev Z, Vadai E, Wensveen F, Ben-Dor S, Golani O, Polic B, Krizhanovsky V. NKG2D ligands mediate immunosurveillance of senescent cells. Aging 2016; 8:328-44; PMID:26878797; http://dx.doi.org/10.18632/aging.100897
- Biran A, Perelmutter M, Gal H, Burton DGA, Ovadya Y, Vadai E, Geiger T, Krizhanovsky V. Senescent cells communicate via intercellular protein transfer. Genes Dev 2015; 29:791-802; PMID:25854920; http://dx.doi.org/10.1101/gad.259341.115
- Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nature Commun 2016; 7:11190; PMID:27048913; http://dx.doi.org/10.1038/ncomms11190
- Chang J, Wang Y, Shao L, Laberge R-M, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding W, Feng W, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 2016; 22:78-83; http://dx.doi.org/10.1038/nm.4010
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015; 14:644-58; PMID:25754370; http://dx.doi.org/10.1111/acel.12344
- Ruhland MK, Loza AJ, Capietto A-H, Luo X, Knoldhoff BL, Flanagan KC, Belt BA, Alspach E, Leahy K, Luo J, et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nature Commun 2016; 7:11762; PMID:27272654; http://dx.doi.org/10.1038/ncomms11762
