Ovarian cancer is the most common cause of death among gynecological cancers due to late presentation and chemotherapy resistance. The High Grade Serous Ovarian Cancer (HGSOC) subtype represents 80% of ovarian tumors and is usually diagnosed at an advanced stage which is already widely metastatic. HGSOC preferentially metastasizes to the abdominal cavity and in particular to the omentum which is a large adipocyte-rich fat pad that covers the small intestine in the abdominal cavity. The establishment of an ovarian cancer metastatic niche is a multi-step process which involves detachment of cancer cells from the site of origin, formation and survival of spheroid-like structures in the peritoneal cavity and re-attachment to fat-rich tissue including the omentum and the peritoneum. The crosstalk between fat cells and cancer cells plays an important role in driving cancer survival and metabolism for several tumor types such as ovarian, breast, prostate and pancreatic cancers.Citation1-3 This is particularly important in the case of ovarian cancer since, unlike other tumors, it rarely metastasizes outside the abdominal cavity. The main cause of death from ovarian cancer is malnutrition secondary to intestinal obstruction because of metastasis-induced adhesion formation. However, the molecular pathways underlying the interaction between adipocytes and ovarian cancer cells have remained poorly understood.
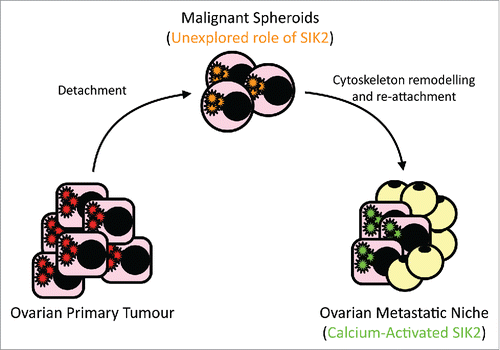
Ahmed et al., previously showed that Salt Inducible Kinase 2 (SIK2) plays an important role in ovarian cancer cell mitosis and survival.Citation4 SIK2 is an AMPK-related protein kinase with an established role in the regulation of cell metabolism, and in re-feeding from starvation.Citation5 More recently we revealed a previously unrecognized role of SIK2 for driving ovarian cancer cell metabolism and proliferation at the adipocyte-rich environment of the abdominal cavity.Citation6 We proposed a key role for SIK2 in establishing abdominal metastases following nutritional deprivation of free-floating cancer cells in the peritoneal cavity (). We found that SIK2 was significantly overexpressed in omental metastases of HGSOCs compared to paired primary lesions. In a xenograft model of ovarian cancer metastasis SIK2-overexpressing cells implanted orthotopically at the ovarian bursa formed significantly larger and more frequent abdominal metastases compared to cells with endogenous levels of SIK2 or cells overexpressing the kinase-inactive SIK2 suggesting that the enzymatic activity of SIK2 is essential for the increased metastatic potential. Depletion of endogenous SIK2 in xenograft models of ovarian cancer significantly reduced ovarian cancer metastasis.Citation6 These findings are important because, they identify SIK2 as a plausible therapeutic target for prevention of ovarian cancer metastasis and, possibly, tumor recurrence.
Figure 1. Ovarian cancer cells become “free-floating” in the peritoneal cavity before they re-attach at the metastatic niche. Cells undergo significant cytoskeletal re-modeling and endure nutrition deprivation before they become established at their metastatic site. Our recent work described how SIK2 activation by adipocytes plays a key role in establishing abdominal metastasis. Future work will explore the possible role of SIK2 in the multi-step metastatic process.
Using an in-cell gate-keeper screen we then identified SIK2-S358 as a key residue for SIK2 autophosphorylation. We extensively validated the SIK2 S358 autophosphorylation residue for use as a readout of SIK2 activity and as a biomarker of on-target activity of SIK2 inhibitors. We then established a system for the co-culture of cancer cells with adipocytes obtained from freshly excised normal omentum from women undergoing surgical staging of ovarian tumors. This system was used in combination with either an ATP-competitive SIK2 kinase inhibitor or siRNA mediated depletion of SIK2 to test the specificity of observed phenotypic effects. Co-culture of ovarian cancer cells with adipocytes resulted in calcium release in ovarian cancer cells and subsequent activation of SIK2 as evidenced by increased S358 phosphorylation. Importantly, SIK2 activation lead to the activation of the PI3K/AKT pathway though phosphorylation of residues S154 and S541 of p85α, the regulatory subunit of the PI3K complex. The link between SIK2 activation and the PI3K complex activation was further confirmed using rapamycin as a known robust paradoxical PI3K activator. We showed that siRNA-mediated depletion of SIK2 or its chemical inhibition significantly reduced rapamycin-induced AKT phosphorylation and sensitized ovarian cancer cells to its cytotoxic effect.Citation6
Importantly, calcium-dependent activation of SIK2 was independent from LKB1 indicating that LKB1 is not a unique regulator of SIK2 but that other kinases may activate it downstream of an increase in intracellular calcium depending on the cell type and context of activation. This is reminiscent of the previously reported activation of AMPK by CAMKK through direct phosphorylation of T172 at the T-loop of the AMPK kinase, a site that is also phosphorylated by LKB1.Citation7
Surprisingly, we also showed that SIK2 augments AMPK in phosphorylating ACC1. This identified SIK2 as an important activator of the fatty acid oxidation pathway.Citation6 These studies suggest that adipocyte-mediated SIK2 phosphorylation and protein stabilization are required to activate cancer cell proliferation and fatty acid oxidation at the omental metastatic niche (). Future work will test whether SIK2 may cooperate with AMPK at the early stages of metastasis when ovarian cancer cells detach from the tumor site of origin. It is also plausible that SIK2 activation is required for cytoskeleton remodeling and re-attachment to the metastatic niche in the abdominal cavity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 2011; 17:1498-503; PMID:22037646; http://dx.doi.org/10.1038/nm.2492
- Laurent V, Guérard A, Mazerolles C, Le Gonidec S, Toulet A, Nieto L, Zaidi F, Majed B, Garandeau D, Socrier Y. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun 2016; 7:10230; PMID:26756352; http://dx.doi.org/10.1038/ncomms10230
- Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov 2016; 6(8):852-69; PMID:27246539
- Ahmed AA, Lu Z, Jennings NB, Etemadmoghadam D, Capalbo L, Jacamo RO, Barbosa-Morais N, Le X-F, Vivas-Mejia P, Lopez-Berestein G, et al. SIK2 is a centrosome kinase required for bipolar mitotic spindle formation that provides a potential target for therapy in ovarian cancer. Cancer Cell 2010; 18:109-21; PMID:20708153; http://dx.doi.org/10.1016/j.ccr.2010.06.018
- Dentin R, Liu Y, Koo S-H, Hedrick S, Vargas T, Heredia J, Yates J, Montminy M. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 2007; 449:366-9; PMID:17805301; http://dx.doi.org/10.1038/nature06128
- Miranda F, Mannion D, Liu S, Zheng Y, Mangala LS, Redondo C, Herrero-Gonzalez S, Xu R, Taylor C, Chedom DF, et al. Salt-inducible kinase 2 couples ovarian cancer cell metabolism with survival at the adipocyte-rich metastatic niche. Cancer Cell 2016; 30(2):273-89; PMID:27478041; http://dx.doi.org/10.1016/j.ccell.2016.06.020
- Woods A, Dickerson K, Heath R, Hong S-P, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2005; 2:21-33; PMID:16054096; http://dx.doi.org/10.1016/j.cmet.2005.06.005
