By acting in cis or in trans, long non-coding RNAs (lncRNAs) take part in numerous cellular processes such as 3D genomic organization, regulation of transcription, splicing, DNA replication and repair, modulation of protein translation and metabolism. Emerging evidence indicates that rather than playing critical roles in these processes, they often act as fine-tuners in cell type specific and/or defined (patho-) physiological conditions.Citation1
This is particularly well-illustrated by recent studies on the lncRNA, NEAT1. The highly conserved NEAT1 locus produces 2 isoforms, the ubiquitous and abundant NEAT1_1 (3.7 kb) and a long isoform, NEAT1_2 (22.7 kb), which functions as the structural component of paraspeckle (PS) nuclear bodies. Expression of NEAT1_2, and thereby PS formation, is restricted to very specific cell types/tissues such as the corpus luteumCitation2 and developing/lactating mammary glands.Citation3 Importantly, mice engineered to lack both NEAT1_1 and NEAT1_2 were viable and fertileCitation4 but exhibited defects in both establishment of pregnancy and lactation. These data indicated that, whereas NEAT1 is dispensable for growth and development, formation of PS, which depends on NEAT1_2, is required under specific physiological conditions. Notably, both of these processes are characterized by high cell proliferation rates, raising the possibility that PS formation may play particularly relevant roles in highly cycling cells.
Consistent with this possibility, we recently demonstrated that PS are assembled in response to oncogenic stress and that lack of NEAT1 compromises the viability of preneoplastic cells.Citation5 When subjected to a skin carcinogenesis protocol, Neat1-deficient mice were, by and large, protected from developing cancer. Concomitant to an overall decrease in cell proliferation and survival, oncogene-expressing Neat1 KO cells exhibited an increased amount of DNA damage and p53 levels. Because oncogenes, through their ability to promote uncontrolled cell proliferation also induce a specific type of DNA damage referred to as replication stress, we postulated that NEAT1_2-dependent PS formation may attenuate the building up of lethal amounts of DNA damage in highly proliferative cells, by coordinating cell proliferation, DNA replication and repair.
NEAT1_2 silencing in established cancer cell lines, which by definition experience oncogene-induced stress led to accumulation of DNA damage. Proper activation of the ATR-Chk1-RPA32 pathway in response to replicative stress is required for cell survival. Strikingly, NEAT1_2 KD cells displayed improper activation of this pathway and thereby of the intra-S-phase checkpoint, which prevents the progression of DNA damaged cells through the cell cycle. Together, these data indicated that NEAT1, through its ability to stimulate PS assembly, promotes ATR–signaling in response to replication stress. Future studies will be required to define the precise molecular mechanisms linking PS formation and proper ATR-CHK1 signaling.
What drives NEAT1_2 expression and PS formation in response to oncogenic stress remains unclear. Interestingly, we found that NEAT1 is a transcriptional target of the tumor suppressor protein p53 and that pharmacological activation of p53 stimulates PS formation. Given that p53 is induced by oncogenic stress, these findings establish the existence of a negative feedback loop that attenuates oncogene–dependent activation of p53 (). It should be mentioned, however, that although attenuated, PS assembly was observed in oncogene-expressing p53-deficient cells. Thus, in addition to p53, other -yet to be defined- pathway(s) also contribute to oncogene-induced PS formation.
Figure 1. NEAT1 modulates DNA-damage response in cancer cells and their sensitivity to genotoxic agents and p53 reactivation therapy. NEAT1-PS and p53 are engaged into a negative feedback loop which attenuates oncogene-induced DNA-damage and p53 activation in pre-neoplastic cells. NEAT1 targeting is synthetic lethal with both genotoxic and p53 reactivating agents.
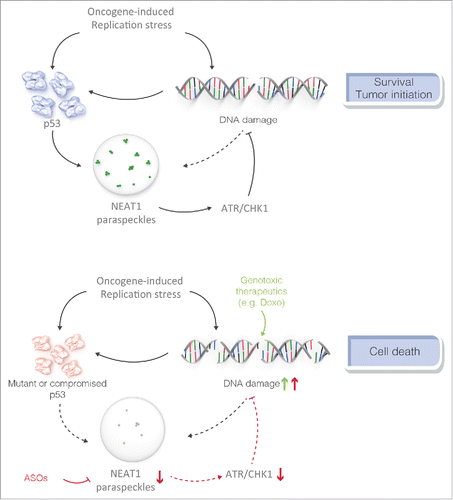
Because Neat1 KO mice do not exhibit characteristic phenotypes associated with deficiency in programmed DSB repair such as growth retardation, progeria or defects in lymphocyte development we conclude that PS are only required for cell survival under very specific physiological conditions and in cells undergoing oncogene-induced replication stress. This intriguing finding offers a unique opportunity to develop therapeutic modalities that are cancer cell specific. On the basis of the deficiency of cancer cells to respond to S-phase checkpoint activation, anticancer therapies could indeed be developed that exacerbate this vulnerability without affecting normal cells. Inhibitors of ATR and CHK1 that selectively kill cancer cells harbouring replication stress are being introduced into the clinic. Similarly, antisense-based agents that target NEAT1 exacerbated the sensitivity of cancer cells to DNA damaging agents (). Consistently, a significant correlation was observed between NEAT1_2 levels and progression free survival time in an ovarian cancer cohort treated with platinium-based chemotherapeutics.
Together, our work establishes a clear genetic and functional link between NEAT1 and cancer and indicates that a wide range of human cancers may benefit from NEAT1 targeting. Our work argues that PS-positive tumors, which based on results obtained using a pan-cancer tissue microarray may represent over 65% of all solid cancer types, are likely to be responsive to such therapeutic strategy. Importantly, however, before NEAT1 therapeutic agents are being introduced into the clinic it will be necessary to define more precisely which cancer types may benefit from NEAT1 therapy. Indeed, the role of modulators of ATR signaling in cancer is ambiguous.Citation6 Haploinsufficiency of the ATR pathway leads to carcinogenesis in mouse models and somatic mutations in ATR and CHK1 have been found in tumors with microsatellite instability.Citation6 Strikingly, mutations within the NEAT1 gene have also recently been described in some human cancers.Citation7 The phenotypic consequences of these mutations on NEAT1 function and the impact of NEAT1 silencing on these particular cancers needs to be further explored.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Li L, Chang HY. Physiological roles of long noncoding RNAs: insight from knockout mice. Trends Cell Biol 2014; 24:594-602; PMID:25022466; http://dx.doi.org/10.1016/j.tcb.2014.06.003
- Nakagawa S, Shimada M, Yanaka K, Mito M, Arai T, Takahashi E, Fujita Y, Fujimori T, Standaert L, Marine J-C, et al. The lncRNA Neat1 is required for corpus luteum formation and the establishment of pregnancy in a subpopulation of mice. Development 2014; 141:4618-27; PMID:25359727; http://dx.doi.org/10.1242/dev.110544
- Standaert L, Adriaens C, Radaelli E, Van Keymeulen A, Blanpain C, Hirose T, Nakagawa S, Marine J-C. The long noncoding RNA Neat1 is required for mammary gland development and lactation. RNA 2014; 20:1844-9; PMID:25316907; http://dx.doi.org/10.1261/rna.047332.114
- Nakagawa S, Naganuma T, Shioi G, Hirose T. Paraspeckles are subpopulation-specific nuclear bodies that are not essential in mice. J Cell Biol 2011; 193:31-9; PMID:21444682; http://dx.doi.org/10.1083/jcb.201011110
- Adriaens C, Standaert L, Barra J, Latil M, Verfaillie A, Kalev P, Boeckx B, Wijnhoven PWG, Radaelli E, Vermi W, et al. p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat Med 2016; 22:861-8; PMID:27376578; http://dx.doi.org/10.1038/nm.4135
- Gaillard H, Garcia-Muse T, Auilera A. Replication stress and cancer. Nat Rev Cancer 2015; 15:276-89; PMID:25907220; http://dx.doi.org/10.1038/nrc3916
- Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB, Martin S, Wedge DC, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 2016; 534(7605):47-54; PMID: 27135926; http://dx.doi.org/10.1038/nature17676
