The balance between hepatic lipogenesis and gluconeogenesis is tightly regulated by complex signaling pathways to retain whole body energy homeostasis. As a key anabolic hormone, insulin promotes de novo lipogenesis to store excessive energy through SREBP1c activation.Citation1 Simultaneously, insulin inhibits hepatic glucose production to prevent unnecessary glucose supply. FOXO1 is one of the key transcription activators that stimulate the expression of gluconeogenic genes including PEPCK and G6Pase during fasting.Citation2 For an acute response, insulin rapidly suppresses hepatic gluconeogenesis through repression of FOXO1 by AKT-mediated phosphorylation. Intriguingly, lipogenic activator SREBP1c has been implicated in the suppression of hepatic glucose production although underlying mechanisms have not been clearly understood.
Our recent study reveals a novel signaling pathway engaging in the inhibition of hepatic gluconeogenesis by SREBP1c.Citation3 In liver, CRY1, one of the circadian clock genes, was identified as a direct target gene of SREBP1c. The expression of CRY1 was upregulated by feeding or insulin through activation of SREBP1c. To investigate whether SREBP1c-dependent CRY1 induction might repress hepatic glucose production, the gluconeogenic capacity of SREBP1c−/− and CRY1−/− mice was assessed by pyruvate tolerance test. Compared to wild type mice, both SREBP1c−/− and CRY1−/− mice showed high levels of blood glucose upon pyruvate challenge. On the other hand, CRY1-overexpresion in SREBP1c-deficient mice resulted in a decrement in pyruvate-induced blood glucose levels. Moreover, ectopic expression of CRY1 reduced the levels of blood glucose in STZ-treated diabetic mice, accompanied with decreases in gluconeogenic gene expression. These results indicate that insulin-induced SREBP1c stimulates CRY1 expression, leading to suppression of hepatic gluconeogenesis.
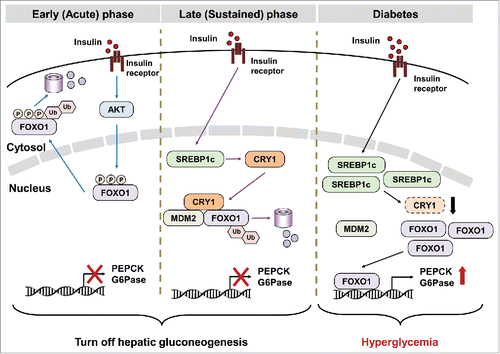
Although CRY1 has been reported as a potential suppressor of gluconeogenesis activated by glucocorticoid receptor and glucagon signaling pathways,Citation4,5 the roles of CRY1 in the regulation of gluconeogenesis under nutrient-rich conditions are largely unknown. In our study, we found that CRY1 accelerated the degradation of nuclear FOXO1 protein in response to insulin. While FOXO1 degradation was rapidly stimulated by AKT-mediated phosphorylation in the early phase of insulin action, CRY1-induced FOXO1 degradation was induced in the late phase of insulin action. Given our observations that CRY1-induced FOXO1 degradation was independent of AKT activity, we propose that CRY1 could sustainably downregulate nuclear FOXO1 protein in late postprandial state.
We also have further investigated factor(s) governing CRY1-dependent FOXO1 degradation. Among several FOXO1 E3 ubiquitin ligases,Citation6 we elucidated that MDM2 participated in CRY1-mediated FOXO1 degradation. CRY1 formed the protein complex with FOXO1 and MDM2, and the physical interaction between FOXO1 and MDM2 was augmented by CRY1. These findings demonstrating that CRY1-mediated FOXO1 degradation was attenuated in the absence of MDM2 prompted us to suggest that increased CRY1 by insulin-activated SREBP1c could stimulate FOXO1 ubiquitination and subsequent degradation by forming MDM2-CRY1-FOXO1 protein complex ().
Figure 1. SREBP1c-CRY1 axis sustainably suppresses glucose production in liver. Insulin suppresses hepatic gluconeogenesis via acute and sustained responses. In early phase of insulin action, AKT-mediated FOXO1 phosphorylation leads to FOXO1degradation in cytoplasm. In addition, insulin sustainably inhibits hepatic gluconeogenesis via CRY1. SREBP1c-mediated CRY1 induction accelerates MDM2-dependent FOXO1 ubiquitination and degradation for prolonged repression of gluconeogenesis. However, the low levels of CRY1 might be one of casual factors for hyperglycemia in diabetic animals.
Hepatic circadian clock gene expression is regulated by day-night cycle as well as feeding-fasting cycle. Moreover, numerous metabolic processes such as gluconeogenesis and lipogenesis are controlled by circadian clock.Citation7 Interestingly, peak time point of CRY1 expression was the same as the trough time point of FOXO1 expression during 24 hours, implying that CRY1 might be involved in the control of gluconeogenesis in not only feeding-dependent but also diurnal circadian dependent manner.
In obese and diabetic animals, insulin fails to inhibit hepatic gluconeogenesis but activates lipogenesis, termed “selective insulin resistance," leading to hyperglycemia as well as hyperlipidemia. Furthermore, increased SREBP1c activity is closely associated with hyperlipidemia in obesity. However, unlike SREBP1c, the level of hepatic CRY1 protein was decreased in obese and diabetic animals. Notably, hepatic CRY1 overexpression relieved hyperglycemia through repressing hepatic gluconeogenic apparatus including FOXO1, G6Pase and PEPCK in db/db mice. Although it remains to be elucidated by which elevated SREBP1c fails to elevate CRY1 protein in obese and diabetic animals, it seems that dysregulation of SREBP1c-CRY1 axis might contribute to cause selective insulin resistance in obesity.
In summary, our findings reveal that insulin effectively governs not only lipogenesis but also gluconeogenesis through SREBP1c activation to maintain whole body energy homeostasis. During postprandial state, insulin is able to sustainably and effectively inhibit the unnecessary glucose production via SREBP1c-mediated CRY1 activation (). In addition, our study elucidates the critical roles of CRY1 in the regulation of gluconeogenesis under physiological and pathological conditions. Therefore, our study suggests that CRY1 might be an attractive therapeutic target to ameliorate hyperglycemia.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Kim JB, Sarraf P, Wright M, Yao KM, Mueller E, Solanes G, Lowell BB, Spiegelman BM. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest 1998; 101:1-9; PMID:9421459; http://dx.doi.org/10.1172/JCI1411
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1–PGC-1 interaction. Nature 2003; 423:550-5; PMID:12754525; http://dx.doi.org/10.1038/nature01667
- Jang H, Lee GY, Selby CP, Lee G, Jeon YG, Lee JH, Cheng KKY, Titchenell P, Birnbaum MJ, Xu A, et al. SREBP1c-CRY1 signalling represses hepatic glucose production by promoting FOXO1 degradation during refeeding. Nat Commun 2016; 7:12180; PMID:27412556; http://dx.doi.org/10.1038/ncomms12180
- Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 2011; 480:552-6; PMID:22170608; http://dx.doi.org/10.1038/nature10700
- Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med 2010; 16:1152-6; PMID:20852621; http://dx.doi.org/10.1038/nm.2214
- Fu W, Ma Q, Chen L, Li P, Zhang M, Ramamoorthy S, Nawaz Z, Shimojima T, Wang H, Yang Y, et al. MDM2 acts downstream of p53 as an E3 ligase to promote FOXO ubiquitination and degradation. J Biol Chem 2009; 284:13987-4000; PMID:19321440; http://dx.doi.org/10.1074/jbc.M901758200
- Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell 2006; 126:801-10; PMID:16923398; http://dx.doi.org/10.1016/j.cell.2006.06.050
