WNT/β-catenin signaling is essential for adult stem cells (ASCs) to maintain tissue homeostasis, regeneration and injury repair.Citation1 As a signaling cascade, a key step is the degradation of β-catenin by the Axin/GSK3β/APC (adenomatous polyposis coli) destruction complex. Cancer genome sequencing has revealed frequent mutations of Wnt pathway components especially APC and CTNNB1 (β-catenin coding gene) in major tumors, highlighting the WNT/β-catenin signaling as an important target of cancer therapy. During the last decade, a number of Wnt inhibitors have been reported but only a few of them have moved to early phase clinical trials. Beside the general obstacles inherent in new drug development, the slow process could be due to the fact that many of these inhibitors target the Wnt signaling at or upstream of the Axin/GSK3β destruction complex, while in cancer patients the major mutant Wnt pathway components (APC and β-catenin) are downstream of that level.
Another challenge in developing Wnt inhibitor for cancer therapy is to avoid the damage to long-term repopulating ASCs that are required for maintaining tissue homeostasis. One opportunity could be the difference between them regarding the type of cell division: ASCs divide asymmetrically under steady-state to maintain population size and tissue homeostasis, while symmetric cell division (SCD) is advantageous for malignant cells to reach excessive expansion and uncontrolled tumor growth.Citation2 In agreement with this idea, emerging evidence indicates the critical role of defective asymmetric division in tumor initiation and progression.Citation2 Particularly, loss of asymmetric division contributes to the oncogenic effect of mutant APC to hyperplasia and tumorigenesis in addition to abnormal activation of Wnt/β-catenin signaling.Citation3 Therefore, enforcing switch of symmetric to asymmetric division in cancer cells potentially allows targeting cancer cells with less damage to normal tissue homeostasis.
In our recent work, we sought to discover Wnt inhibitors by screening clinical approved drugs with well characterized bioactivity and safety profiles.Citation4 Axitinib, a tyrosine receptor kinase inhibitor approved for treatment of renal cell carcinoma,Citation6 showed strong inhibition of Wnt signaling activated by GSK3 inhibitor 6-bromoindirubin-3’-oxime (6BIO) and β-catenin lacking the N-terminal in culture cells. In addition, Axitinib inhibited Wnt signaling and promoted β-catenin turnover in SW480 cancer cells harboring APC with mutation commonly detected in cancer patients, supporting that Axitinib blocks the Wnt/β-catenin signaling downstream and independent of the GSK3β/APC destruction complex. We also evaluated the Wnt inhibition in zebrafish models. In fish embryos Axitinib dose dependently rescued the eyeless phenotype induced by abnormal activation of Wnt/β-catenin signaling, and strongly inhibited the tailfin regeneration and Wnt signaling activation in adult TCF-GFP transgenic fish, directly demonstrating that Axitinib inhibits Wnt/β-catenin signaling in vitro and in vivo.
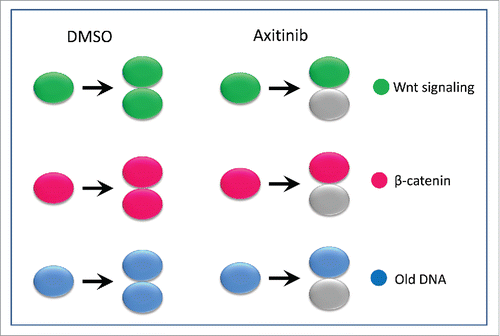
An intriguing observation in Axitnib treated SW480 cancer cells is the unequal Wnt signaling and β-catenin distribution in paired cells, indicating the asymmetric cell division (ACD) which was rarely observed in control SW480 cells. Notably, in Axitinib treated cells we also observed non-random DNA segregation that is a well-established ACD feature of stem cells().Citation5 Correlation analysis revealed that Axitinib induced non-random DNA segregation is dependent on the β-catenin degradation (). Given that loss of ACD is critical for tumor initiation and progression,Citation2 re-establishment of ACD in cancer cells could be a mechanism of tumor suppression and an opportunity to selectively target tumor cells with less effect on adult tissue homeostasis. For normal ASCs dividing symmetrically in a neutral drift process, the direction of ACD by Axitinib will potentially allow the ASCs to continuously maintain the stem cell pool. However, ASCs will be affected by Axitinib in case SCD is required to expand the stem-cell pool following injury or disease,Citation2 as shown by the inhibited tailfin regrowth in adult zebrafish.
Figure 1. Axitinib induces asymmetric cell division in terms of Wnt signaling, β-catenin and DNA segregation. Gray marks cells losing Wnt signaling, β-catenin or old DNA during cell division. All the gray cells in the bottom indicates that cells loss of Wnt/β-catenin consistently loss old DNA.
Axitinib is a known inhibitor of multi-receptor tyrosine kinases, especially vascular endothelial growth factor receptors (VEGFRs).Citation6 However, these established tyrosine receptor kinase targets are silenced in SW480 cells. In addition, 3 other VEGFR inhibitors did not show Wnt inhibition, suggesting that Axitinib inhibits Wnt signaling via targets other than VEGFRs. To identify the direct binding proteins of Axitinib, we combined DARTS (drug affinity responsive target stability) and 2D-DIGE (2-dimensional difference in gel electrophoresis) and mass spectrometry methods, and demonstrated an E3 ubiquitin ligase SHPRH (SNF2, Histone-Linker, PHD and RING finger domain-containing Helicase) as the direct target of Axitinib in Wnt inhibition. Overexpression of SHPRH increased the ubiquitination/degradation of β-catenin and decreased Wnt signaling in SW480 cells, supporting SHPRH as a novel regulator of Wnt/β-catenin signaling. The discovery of SHPRH as the direct and functional target of Axitinib strongly supports the notion that although drugs are intended to be selective, many effective drugs bind to multiple molecules rather than single targets, and this “polypharmacology” is probably therapeutically essential.Citation7 Therefore, repurposing of clinical approved drugs could be a strategy to accelerate the process of drug discovery and development.
Disclosure of potential conflicts of interest
Use of axitinib in β-catenin inhibition is covered by United Kingdom Patent Application No. 1519258.6 in which XK, YQ and KHK are inventors.
References
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature 2005; 434:843-50; PMID:15829953; http://dx.doi.org/10.1038/nature03319
- Bajaj J, Zimdahl B, Reya T. Fearful Symmetry: Subversion of asymmetric division in cancer development and progression. Cancer Res 2015; 75:792-797; PMID:25681272; http://dx.doi.org/10.1158/0008-5472.CAN-14-2750
- Quyn AJ, Appleton PL, Carey FA, Steele RJC, Barker N, Clevers H, Ridgway RA, Sansom OJ, Näthke IS. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell 2010; 6:175-181; PMID:20144789; http://dx.doi.org/10.1016/j.stem.2009.12.007
- Qu Y, Gharbi N, Yuan X, Olsen JR, Blicher P, Dalhus B, Brokstad KA, Lin B, Øyan AM, Zhang W, et al. Axitinib blocks Wnt/β-catenin signaling and directs asymmetric cell division in cancer. Proc Natl Acad Sci U S A 2016; 113:9339-44; PMID:27482107; http://dx.doi.org/10.1073/pnas.1604520113
- Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol 2007; 5:e102; PMID:17439301; http://dx.doi.org/10.1371/journal.pbio.0050102
- Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu EY, et al. Nonclinical antiangiogenesis and antitumor activities of Axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res 2008; 14:7272-7283; PMID:19010843; http://dx.doi.org/10.1158/1078-0432.CCR-08-0652
- Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol 2008; 4:682-690; PMID:18936753; http://dx.doi.org/10.1038/nchembio.118
