Cells employ multiple mechanisms to maintain a delicate balance of protein synthesis, folding, and degradation, collectively termed proteostasis.Citation1 Disrupting proteostasis through proteasome inhibition has yielded beneficial effects in multiple myeloma, but few other cancers.Citation2 This observation suggests that cancer cells may harbor context-specific dependencies on proteostasis machinery, and opens an opportunity for precision therapy informed by cell biology.
To uncover and dissect such cancer-specific dependencies, we tested a chemical inhibitor of heat shock protein-70 kgDalton (HSP70) chaperones, MAL3-101, in a panel of patient-derived cancer cell lines.Citation3 Members of the HSP70 family assist nascent and misfolding proteins to properly fold or target them for degradation, making them important guardians of proteostasis.Citation4 To our surprise, cell lines derived from the myogenic tumor rhabdomyosarcoma (RMS) were uniformly more sensitive to HSP70 inhibition than any other line tested. RMS tumors are classified into 2 categories based on their histology and genomics: alveolar tumors harbor the recurrent PAX3-FOXO1 translocation, whereas embryonal tumors are frequently found to have activating mutations in the MAP kinase pathway.Citation5 Intriguingly, HSP70 inhibition demonstrated equivalent effects in either background, suggesting that dependence on this chaperone in RMS is not determined by a single genomic event, but instead is a broader feature of this ontogeny.
Through transcriptome sequencing, we identified that activation of the unfolded protein response (UPR) was a hallmark of RMS cells following HSP70 inhibition. The UPR is a conserved set of signaling pathways that transmit information on the state of protein folding within the endoplasmic reticulum (ER) to the rest of the cell.Citation1 We demonstrated genetically that the terminal UPR effector CHOP, whose transcription and translation are both enhanced by activation of the UPR sensor PERK, was necessary for apoptosis following HSP70 inhibition. These data demonstrate that RMS cells are especially susceptible to failure of protein quality control within the ER, as this activates a pro-apoptotic UPR signal.
There are 14 human HSP70 isoforms with distinct localizations and behaviors.Citation4 Unexpectedly, targeted abrogation of cytosolic and not ER-localized HSP70 was sufficient to recapitulate the drug’s effects, leading to the surprising conclusion that RMS cells rely on a cytosolic chaperone to silence lethal ER stress.
Taken together, our findings highlight an exciting new opportunity for targeted therapy in a disease with extremely poor survival in the metastatic setting. Future preclinical testing of this agent and other cytosolic HSP70 inhibitors will serve as a prelude to clinical trials. However, a deeper molecular understanding of the basis for HSP70 dependence in RMS would enhance the impact of such trials by identifying the specific patients, both with RMS or other cancers, who stand to gain the most by this approach. One chief question needs to be answered in the laboratory to enable biomarkers of HSP70 inhibitor response: what molecular features of RMS dictate dependence on HSP70 to enable cell survival?
One possibility is that RMS cells express a specific lineage factor that is an obligate HSP70 client, and that loss of critical chaperone activity leads to rapid misfolded substrate accumulation and catastrophic UPR activation. Many human diseases including retinal dystrophies are characterized by apoptosis resulting from the misfolding of a single mutated client.Citation6 Further, some inherited myopathies arise from misfolding of structural myogenic elements with subsequent UPR activation,Citation7 nominating potential “bad seeds” that RMS cells are endowed with as part of their aberrant differentiation program. If this is the case, identifying tumors with high expression of this HSP70 client will be critical, as they would have the most robust effects of chaperone inhibition ().
Figure 1. Models for RMS HSP70 dependence. Top, the RMS ER carries a specific HSP70 client that misfolds after treatment, activating the UPR. Client-expressing tumors are sensitive to HSP70 inhibition. Below, the RMS UPR is “primed” at rest and slight perturbation by HSP70 inhibition reaches apoptotic threshold. HSP70 inhibitors are predicted to be most effective in cells with high resting UPR sensitivity.
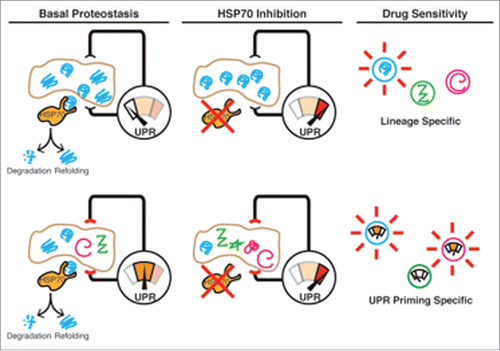
An alternative, but not mutually exclusive notion is that the ER stress networks of RMS cells are intrinsically biased toward apoptosis. Thus even minor perturbations in protein folding in the “toxic soil” of the RMS ER may lead irrevocably to apoptosis. One potential example of this is the recurrent 12q13-14 amplification seen in some RMS patients, which includes the genomic locus for CHOP and may thus prime the UPR. In this model, UPR priming is the most important biomarker of HSP70 dependence, which may be assessed through transcriptomic or proteomic interrogation of biopsies ().
Broadly, our work confirms that knowledge of cancer-specific wiring of protein homeostasis networks can inform disease mechanism and create opportunities for precision cancer therapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Brodsky JL, Skach WR. Protein folding and quality control in the endoplasmic reticulum: Recent lessons from yeast and mammalian cell systems. Curr Opin Cell Biol 2011; 23:464-75; PMID:21664808; http://dx.doi.org/10.1016/j.ceb.2011.05.004
- Dou QP, Zonder JA. Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system. Curr Cancer Drug Targets 2014; 14:517-36; PMID:25092212; http://dx.doi.org/10.2174/1568009614666140804154511
- Sabnis AJ, Guerriero CJ, Olivas V, Sayana A, Shue J, Flanagan J, Asthana S, Paton AW, Paton JC, Gestwicki JE, et al. Combined chemical-genetic approach identifies cytosolic HSP70 dependence in rhabdomyosarcoma. Proc Natl Acad Sci U S A 2016; 113:9015-20; PMID:27450084; http://dx.doi.org/10.1073/pnas.1603883113
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 2005; 62:670-84; PMID:15770419; http://dx.doi.org/10.1007/s00018-004-4464-6
- Shern JF, Chen L, Chmielecki J, Wei JS, Patidar R, Rosenberg M, Ambrogio L, Auclair D, Wang J, Song YK, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov 2014; 4:216-31; PMID:24436047; http://dx.doi.org/10.1158/2159-8290.CD-13-0639
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science 2007; 318:944-9; PMID:17991856; http://dx.doi.org/10.1126/science.1146361
- Sandonà D, Betto R. Sarcoglycanopathies: molecular pathogenesis and therapeutic prospects. Expert Rev Mol Med 2009; 11:e28; PMID:19781108; http://dx.doi.org/10.1017/S1462399409001203
