Amyloids (insoluble β-sheet polymers) and prions (proteinaceous infectious particles) have long been known for their roles in neurodegenerative diseases such as Parkinson Disease (PD) and Alzheimer disease (AD), as well as rare prion diseases. Amyloid and prion proteins adopt alternative conformations; they are typically expressed as soluble monomers, but can convert to an insoluble, aggregate state. The aggregate state is self-promoting and can convert the soluble protein so that it adopts the alternative, aggregating form. In disease states, the aggregating form prevails and may lead to pathologies associated with AD, PD and prion diseases.Citation1 The majority of our knowledge about prions and amyloids comes from the study of pathogenic protein aggregates found in these diseases. However, the role of amyloids in physiological processes has been understudied. Recently, a number of amyloid- and prion-like proteins have been identified with various physiological functions, which include roles in necrosome formation,Citation2 innate immune signaling in mammalsCitation3 and gametogenesisCitation4 in yeast. Here, we discuss our recent work showing how physiological amyloids can be involved in preserving dormancy in vertebrate oocytes, by forming a subcellular compartment, called the Balbiani body.Citation5
The Balbiani body is a non-membrane bound compartment packed with mitochondria, E.R, Golgi, and RNA.Citation5 It is present in the cytoplasm of all vertebrate oocytes in which it has been looked for. The Balbiani body is a transient structure, as it only exists in the dormant oocytes, and disperses once the oocyte is activated.Citation6 We will term this assembly a “compartment” since its protein, nucleic acid and organelle composition clearly differ from the bulk cytoplasm of the oocyte, and it has a sharp boundary. It differs from conventional compartments like E.R, nucleus and Golgi, in not being contained by a lipid bilayer. Therefore, some other physical principle must cause its formation and prevent its contents mixing with the bulk cytoplasm.
We have studied Balbiani bodies in the giant oocytes of Xenopus laevis frogs. The size advantage of Xenopus made it possible to isolate the Balbiani body, and determine its macromolecular components using proteomics and RNA-seq. A protein called Velo1, or Xvelo, is one of the most enriched proteins in the Balbiani bodies of Xenopus. Xvelo localizes to the Balbiani body in early stage oocytes, and fills the gaps between the mitochondria. Moreover, photobleaching of Xvelo-GFP localized in Balbiani bodies suggested that Xvelo forms a stable matrix in the Balbiani body. Pure protein experiments demonstrated that Xvelo forms micron scale networks in vitro, which can bind to RNA and recruit mitochondria in a cell-free system. We concluded that Xvelo self-assembly drives the Balbiani body formation and an Xvelo matrix is the glue that keeps the components of Balbiani body together.Citation5
How can a protein form a stable, micron-scale, organelle embedding matrix in the cytoplasm? The answer proved to be an unexpected link between oocyte biology and amyloid and prion concepts.
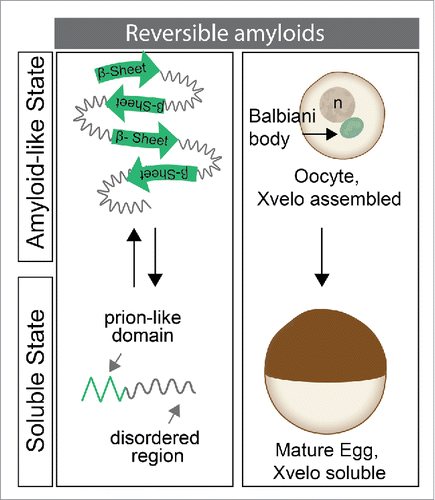
Xvelo is a disordered protein with no conventional domains. Orthologues of Xvelo in other species, like Bucky Ball in zebrafish, are also disordered. However, we found that a conserved feature of all known Balbiani body organizers is that they have a predicted prion-like domain (PLD).Citation5,7 These regions are aggregation-prone and tend to be enriched in aromatic amino acids and depleted in charged amino acids. Upon stimuli, they have the capacity to form amyloids, which have inherent and highly efficient self-templating capacity.Citation7
Xvelo networks exhibit amyloid-like properties, both as pure protein and in oocytes, as detected by their SDS resistance, dye binding and electron microscopy studies (). When we introduced the PLD of Xvelo into the oocytes alone, it was sufficient for targeting to the Balbiani body, suggesting it interacts and assembles with the endogenous Xvelo matrix in Balbiani bodies. Mutating the aromatic residues of the PLD of Xvelo to a charged residue, aspartate, interfered with and abolished the ability of Xvelo to assemble into amyloids both in vivo and in vitro. Moreover, the only other protein that was able to localize to and form a stable matrix in the Balbiani body was the zebrafish homolog of Xvelo, Bucky ball.Citation5 This suggests that information in the PLDs of these proteins is conserved and required for their assembly into amyloid-like networks to form the Balbiani body. We have also shown that Xvelo does not interact with random proteins with a PLD in vivo and in vitro, suggesting the self-assembly process is highly specific.Citation5
Physiological amyloids are attractive to study because, in contrast to the pathological forms, they are reversible. The pathological amyloids are highly stable and it has been difficult to develop therapies that dissolve the aggregates in diseases like ALS, AD or PD.Citation1 Balbiani bodies are transient structures; they disappear upon receiving maturation signals. How they dissociate will be the focus of future work; however, our preliminary data hint that the phosphorylation status of Xvelo may play an important role in this process. Further studies on this mechanism may give us important insights into understanding the nature of pathological amyloids, and suggest mechanisms for their dissociation.
Figure 1. Amyloid-like assembly of Xvelo is regulated in oogenesis. In early stage oocytes, Xvelo self-assembles to drive formation of the Balbiani body and displays amyloid-like properties. As the oocyte matures, Balbiani body disperses. Although Xvelo is still present in mature oocytes, called eggs, it does not show amyloid-like characteristics.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Goedert M. Alzheimer's and Parkinson's diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 2015; 349(6248):1255555; PMID: 26250687; http://dx.doi.org/10.1126/science.1255555
- Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao Y-S, Damko E, Moquin D, Walz T, McDermott A, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 2012; 150, 339-50; PMID:22817896; http://dx.doi.org/10.1016/j.cell.2012.06.019
- Hou F, Sun L, Zheng H, Skaug B, Jiang Q-X, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 2011; 146(3):448-61; PMID: 21782231; http://dx.doi.org/10.1016/j.cell.2011.06.041
- Berchowitz LE, Kabachinski G, Walker MR, Carlile TM, Gilbert WV, Schwartz TU, Amon A. Regulated formation of an amyloid-like translational repressor governs gametogenesis. Cell 2015; 163, 406-18; PMID: 26411291; http://dx.doi.org/10.1016/j.cell.2015.08.060
- Boke E, Ruer M, Wühr M, Coughlin M, Lemaitre R, Gygi SP, Alberti S, Drechsel D, Hyman AA, Mitchison TJ. Amyloid-like self-assembly of a cellular compartment. Cell 2016; 166(3):637-50; PMID: 27471966; http://dx.doi.org/10.1016/j.cell.2016.06.051
- Pepling ME, Wilhelm JE, O'Hara AL, Gephardt GW, Spradling AC. Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc Natl Acad Sci USA 2007; 104(1):187-92; PMID:17189423; http://dx.doi.org/10.1073/pnas.0609923104
- Malinovska L, Kroschwald S, Alberti S. Protein disorder, prion propensities, and self-organizing macromolecular collectives. Biochim Biophys Acta. 2013; 1834(5):918-31; PMID: 23328411; http://dx.doi.org/10.1016/j.bbapap.2013.01.003
