ABSTRACT
Mutations in Klp10A, a microtubule-depolymerising Kinesin-13, lead to overly long centrioles in Drosophila male germ cells. We demonstrated that the loss of Klp10A modifies the distribution of typical proteins involved in centriole assembly and function. In the absence of Klp10A the distribution of Drosophila pericentrin-like protein (Dplp), Sas-4 and Sak/Plk4 that are restricted in control testes to the proximal end of the centriole increase along the centriole length. Remarkably, the cartwheel is lacking or it appears abnormal in mutant centrioles, suggesting that this structure may spatially delimit protein localization. Moreover, the parent centrioles that in control cells have the same dimensions grow at different rates in mutant testes with the mother centrioles longer than the daughters. Daughter centrioles have often an ectopic position with respect to the proximal end of the mothers and failed to recruit Dplp.
Introduction
The centrioles are cylindrical structures formed by singlet, doublets or triplets microtubules usually arranged in a beautiful ninefold symmetry.Citation1,2 These organelles are instrumental to the assembly of a functional centrosome since they play crucial roles to spatially organize the proteins involved in the recruitment of γ-TuRC complexes need to nucleate the microtubule network during interphase and mitosis.Citation3-5 Therefore, decipher the centriole structure and behavior during the cell cycle is crucial to understand the centrosome function and dynamics.
The knowledge of the molecular mechanisms of centriole assembly and centriole-to-centrosome conversion have greatly increase in the last years,Citation5-9 and super-resolution immunoflorescence microscopy techniques greatly help us to decipher the localization of the main centriole-associated proteins involved in centriole organization and PCM recruitment in both Drosophila and human somatic cells.Citation10-13
The somatic centrioles are useful models to look at the dynamics of the centriole-associated proteins during progression through the cell cycle and centrosome maturation.Citation5 However, they are too short to appreciate in detail eventual differences of protein localizations along their length. To circumvent these issues we focused our attention on centriole-associated proteins during Drosophila male gametogenesis where the centriole length increases significantly.
The centrioles of the germ line stem cells and spermatogonia in the Drosophila testis look like centrioles of somatic cells in that they are short and duplicate once during each cell cycle in concert with DNA replication. However, germ line centrioles in Drosophila are composed by triplet microtubules whereas the somatic centrioles have doublets.Citation14,15 Interestingly, all centrioles in the Drosophila tissues retain their cartwheel. By contrast, the cartwheel is only transiently present within the daughter centrioles of vertebrate cells and it is lost during daughter-mother transition at the onset of mitosis.Citation16 Moreover, there is a clear structural asymmetry between the parent centrioles of germline stem cell. The mother one that is localized close to the hub region is composed of triplets, whereas the daughter displays mixed doublets and triplets and will acquire the full triplet number later.Citation15
Early spermatocytes inherit at the end of the fourth spermatogonial mitosis 2 orthogonally arranged centrioles that duplicate and move to the cell periphery to organize distinct cilium-like projections that persist through the meiotic divisions.Citation17-19 Thus, each primary spermatocyte displays 4 ciliary processes suggesting that all the centrioles have the same competence.Citation20 This is in contrast with the prevailing view that only the mother centriole is able to nucleate a ciliary axoneme that is disassembled in vertebrate cells at the end of the interphase.Citation21,22
During the first prophase the cilium-like regions grow by an unidentified mechanism independent by the intraflagellar transport and the centrioles elongate concurrently to reach a 10-fold length at the onset of prometaphase.Citation23 This aspect contrasts with the findings that the basal bodies of ciliated cells do not increase dimensions during ciliogenesis. Since the distal region of the spermatocyte centrioles is engaged in the assembly of the ciliary axoneme it is still unclear how the centriole may elongate. Therefore, the process driving centriole growth in Drosophila spermatocytes does not simply relies on the molecular pathways involved in centriole assembly and elongation in other organisms.Citation24
Klp10A, a microtubule-depolymerising Kinesin-13, involved in microtubule dynamics during both interphase and mitosis,Citation25-27 seems to play a major role in the dynamics of the Drosophila centriole. Loss of Klp10A leads, indeed, to uncontrolled centriole length and significant instability in germ line and somatic Drosophila cells.Citation28,29 However, it is still unclear how this protein works to control centriole length. To better understand the role of Klp10A on centriole structure and add insights into the mechanism of PCM recruitment we asked whether the localization of Dplp and Spd2, 2 centriole associated proteins critical for PCM organization,Citation3,6,30,31 and Sak-Plk4 and Sas-4, essential to centriole duplication and assembly,Citation24,32-34 may be affected in overly long centrioles of mutant cells.
We show here that Klp10A, beyond its established role in centriole growth during the spermatogonial divisions and in young primary spermatocytes, is differently required by parent centrioles to achieve their full length. We found, indeed, that mother centrioles are longer than daughters after Klp10a loss. Moreover, we also observe that Dplp, Sas-4 and Sak/Plk4 have a broad localization along the length of the mutant centrioles, whereas in control germline cells these proteins are restricted to the proximal regions of the centrioles.
Results
Parent centrioles have different length in Klp10A mutant testes
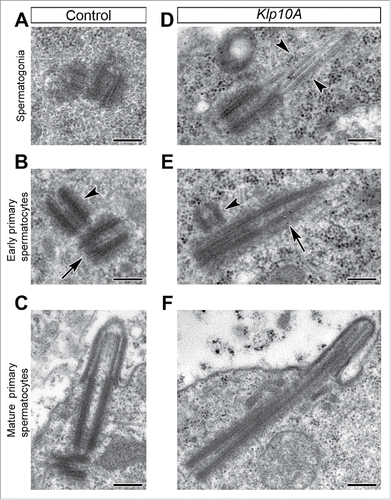
The Drosohila centrioles are short in stem cells and spermatogonia () and elongate in young primary spermatocytes () to reach their full length at the end of the first prophase (). Loss of Klp10A leads to uncontrolled centriole elongation and significant centriole instability. Centrioles were unusually long during the last spermatogonial division () and in young primary spermatocytes () and often displayed growing microtubule bundles at their distal ends () as previously observed in Klp10A wing disc cells.Citation29 Unlike control tests in which the parent centrioles have the same size, the mother centrioles in mutants were more elongated than daughters (). Moreover, the position of the daughter centrioles respect to the mother ones was unusual: in control testes the procentrioles always formed at the proximal end of the mothers, whereas in mutant testes the daughter centrioles were displaced away from the proximal end of the mothers (). The centrioles that reached the cell surface assembled abnormal and short cilium-like projections ().
Figure 1. Klp10A loss leads to overly long centrioles. Centrioles in control (A–C) and mutant (D–F) testes. (A–C) Centrioles elongate gradually in control testes and reach the full length at the end of the first prophase when their distal end organizes a cilium-like projection. Mother (B, arrow) and daughter (B, arrowheads) centrioles have the same length in control germ cells. Mutant centrioles are unusually long with blurred distal ends that often prolonged in single or doublet tubules (D,E, arrowheads); the daughter centrioles (E, arrowhead) are shorter than the mothers (E, arrow) and are displaced from the proximal end of the mothers. Scale bars: 250 nm.
Klp10A mutation affects the localization of Dplp
To analyze the centriole-associated protein dynamics during male gametogenesis we firstly look at the localization of Dplp that in cultured S2 cells is tightly associated with the centriole wall where it organizes a fibrillar network to scaffold centrosomal proteins around the centriole.Citation12 To better understand the distribution of Dplp we counterstained control and mutant testes with an antibody against the PCM protein Spd-2 that recognized the whole Drosophila centrioles. In control testes, Dplp localized on the whole centrioles of stem cells and spermatogonia where it formed a distinct ring around Spd-2 (). In late prophase spermatocytes the Dplp staining was restricted at the basis of the giant centrioles ().Citation10,19
Figure 2. The recruitment of Dplp increases in Klp10A mutant testes. Low magnification of the hub region and early primary spermatocytes in control (A) and mutant (C) testes; centrioles are dot-like in control germline stem cells and early primary spermatocytes, whereas they are very elongated in mutant germ cells (Spd-2, green; Dplp, red; DNA, blue; GSCs, germline stem cells). Representative centrioles in control (B) and mutant (D) male germ cells at indicated stages of development were stained to reveal Spd-2 (green) and Dplp (red). Dplp forms in control testes a short cylinder (B, arrowheads) that elongates in mutant centrioles (D, arrows) and surrounds Spd-2. The paired signals found in mature primary spermatocytes correspond to V-shaped pairs of centrioles. Mother centrioles (D, m) are longer than daughters (D, d) and stained for Spd-2 and Dplp, whereas during the earlier stages of spermatogenesis the daughters often stained only for Spd-2. Acetylated-tubulin labeling (green) reveals distinct cilium-like projections in control mature primary spermatocytes (E, arrowheads) but not during the earlier phases of spermatogenesis in mutant testes; centrioles are stained with Spd-2 (red); GSCs, germline stem cells.Scale bars: A,C = 5 µm; B,D,E,F = 1 µm.
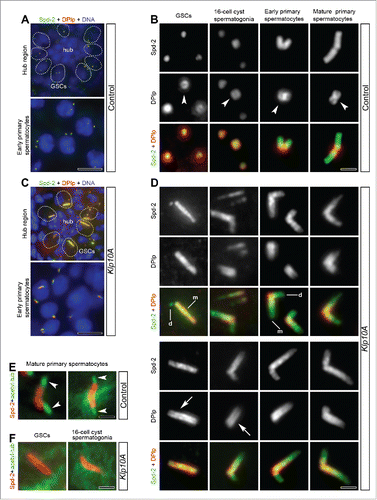
The more dramatic effects of Klp10A loss were observed during the early stages of male gametogenesis. Unusually long centrioles were, indeed, found in germline stem cells, in 16-cell cysts of spermatogonia and in young primary spermatocytes (). The localization of Dplp was highly perturbed and acquired an expanded distribution along the centriole length ().
To exclude the possibility that the overly long centrioles found in germline stem cells and spermatogonia may be the result of the premature assembly of cilium-like projections, we counterstained mutant testes with antibodies against acetylated α-tubulin that recognize the stable microtubules within the ciliary projections of Drosophila spermatocytes ().Citation19,35 However, this antibody failed to recognize such structures at the distal ends of the elongated centrioles in mutant germ cells ().
Both electron microscopy (EM) and immunofluorescence analysis showed that the parent centrioles had the same length in control testes ( and). By contrast, in mutant germ cells most of the daughter centrioles examined (201 out of 236) were shorter than mothers ( and). This was dramatically evident in germ line stem cells where we find overly long mother centrioles (48 out 97) close to the hub region, whereas their daughters always appeared as small spots (). We also find that the daughter centrioles of mutant 16-cell cyst spermatogonia and young primary spermatocytes in some cases (39 out 85) lack Dplp signal whereas the mother centrioles stained for both Dplp and Spd-2 (). This asymmetric localization was not longer seen in mature primary spermatocytes where both parents display a distinct Dplp signal ().
Sas-4 is not required to maintain Dplp and Spd-2 at the centriole during meiosis
In fly somatic cells the PACT domain of Dplp is close to the centriole wall, where Sas-4 links the cartwheel to the peripheral microtubules promoting their elongation during centriole assembly.Citation12,36-38 We then asked whether there was a correlation between Dplp and Sas-4. Sas-4 co-localized with Dplp and Spd-2 on the wall of the centrioles in somatic and in male germline stem cells ().Citation10 The distribution of Sas-4 did not change significantly in mature primary prophase spermatocytes despite the centrioles had elongated 10 times (). Sas-4 was restricted to the proximal region of the giant centrioles () in correspondence of the Dplp staining (). However, although, Dplp and Sas-4 signals overlap at the basis of the centrioles, their specific localization differs with Dplp forming a thick cylinder around Sas-4 (). All the centrioles of the 16-cell cyst spermatogonia displayed a distinct Sas-4 signal, whereas one centriole of the pairs often lacks Spd2 and Dplp ().
Figure 3. Sas-4 distribution in Klp10A depleted testes. Centrioles in control (A,B) and mutant (C,D) male germ cells at different stages of development were stained to reveal Sas-4 (red), Dplp (green, left column) and Spd-2 (green, right column). (A,B) Dplp and Spd-2 co-localize with Sas-4 during the spermatogonial mitoses although one of the just duplicated centrioles within each pair often lacks Dplp and Spd-2 signals. In mature primary spermatocytes the Sas-4 signal co-localizes with Dplp (A) and is found at the proximal end of the centrioles fully stained by the anti-Spd-2 antibody (B). In Klp10A mutant testes the Sas-4 localization extends over Dplp (C) and Spd-2 (D) signals in 16-cell cyst spermatogonia. (C,D) In mature primary spermatocytes the Sas-4 signal shrinks. Scale bars: 1 µm.
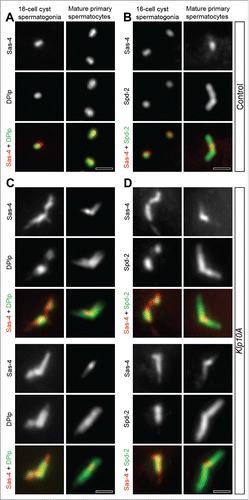
We reasoned that if there was interdependence between the localization of Sas-4 and Dplp, then an increase of Sas-4 signal in Klp10A mutants must correspond to similar changes in Dplp distribution. We find, indeed, that the centrioles and centriole fragments were strongly stained by both anti-Sas-4 and anti-Dplp antibodies in 16-cell cyst spermatogonia and in young primary spermatocytes (). However, Sas-4 was present along the entire length of the abnormal centrioles in these earlier stages, whereas the Dplp signal did not exceed their proximal half (). The localization of Sas-4 during the last spermatogonial mitoses and in early primary spermatocytes also extended beyond the region occupied by Spd-2 along the abnormal centrioles (). However, the intensity of the Sas-4 signal in mutant testes decreased as prophase progressed and became restricted to the proximal region of the centrioles in mature spermatocytes (). By contrast, Dplp and Spd-2 maintained a broad localization.
The proximal region of the centriole is a distinct domain
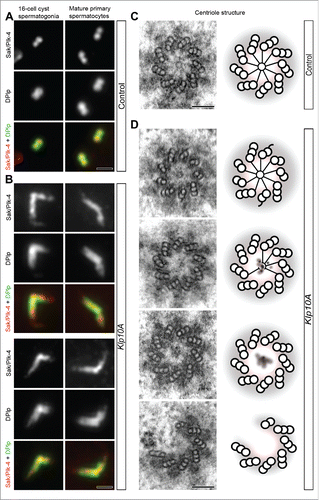
The unusual position of the daughter centrioles placed away from the proximal ends of the mothers in mutant testes () prompted us to verify if Klp10A loss may also affect the localization of Plk4 (Polo-like kinase 4) that plays a main role in the assembly of the procentrioles and in the sequential recruitment of some centriole-associated proteins.Citation32,33,39,40 Plk4 colocalized in control testes with Dplp at the proximal end of the centrioles () where also Sas-4 was found. Plk4 was not restricted to the proximal region of the centrioles in 16-cell cysts of the spermatogonia, and in early and mature primary prophase spermatocytes but extended beyond this region inside an elongated Dplp cylinder (). Remarkably, Plk4, Dplp and Sas-4 signals roughly matched in control testes the cartwheel position at the basis of the centriole. By contrast, the centrioles examined in Klp10A mutant spermatogonia and young primary spermatocytes rarely had a distinct cartwheel ( 9 out of 67) as that found in control cells (). Rather, mutant centrioles lack the cartwheel ( 25 out of 67) or it was abnormal, with indistinct hub and incomplete spokes ( 33 out of 67). Plk4, Dplp and Sas-4 were also localized on the whole centriole fragments that lack cartwheel components frequently found in young primary spermatocytes.
Figure 4. The proximal region of the centriole. Centrioles in control (A) and mutant (B) male germ cells were stained to reveal Sak/Plk4 (red) and Dplp (green). Sak/Plk4 and Dplp co-localize in control testes to the short spermatogonial centrioles or at the proximal end of the giant primary spermatocyte centrioles (A), but expand along the abnormal centrioles in mutant testes (B). Cross sections through the proximal end of control (C) and mutant (D) centrioles: a distinct cartwheel is visible in control centrioles, whereas it is rarely found in mutant centrioles that often lack a cartwheel or display blurred cartwheels. Cartoons depicting the architecture of the basal region of control (C) and mutant (D) centrioles. Scale bars: A,B = 1 µm; C,D = 100 nm.
Discussion
The centrosome in vertebrate cells contains one pair of centrioles with distinctive morphologies and functions.Citation41 The mother centriole displays characteristic distal appendages involved in microtubule binding and assembles the primary cilium.Citation42 By contrast, the parent centrioles in Drosophila lack a distinct structural dimorphism and both nucleate a cilium-like projection although they maintain the cartwheel that represents a hallmark of immaturity.Citation23,43 Thus, they may be only recognized by their reciprocal position with the daughter centriole orthogonal to the proximal end of the mother.
The parent centrioles have a different length in the absence of Klp10A in 16-cell cysts of the spermatogonia and in young primary spermatocytes. The mother centrioles, indeed, are much longer than daughters. This could be explained with the role of Klp10A on centriole stability.Citation28 Klp10A loss leads, indeed, to centriole fragmentation. Therefore, the daughter might be more instable than the mother during the elongation process and, therefore, it could undergo to a delayed growth. Accordingly, Dplp is always associated in mutant testes to the mother centriole, but it is recruited to the daughters later when they have already duplicated and are growing. By contrast, in control testes Dplp is always present on parent centrioles. Thus, Klp10A seems to play opposite roles in the dynamic of the parent centrioles, allowing the elongation of the mother, but delaying the growth of the daughter. This observations point to a diverse requirement for Klp10A in the behavior of the parent centrioles thus uncovering new intrinsic structural dimorphisms that was not morphologically apparent during normal development.
Since the reduced length of the daughter centrioles is associated with the failure to recruit Dplp, an intriguing hypothesis is that Dplp could play some role in centriole elongation likely due to its involvement in the recruitment of centriole-associated proteins. Depletion of Dplp affects the recruitment of interphase PCM components in fly cultured cells, and its association with Cnn is required to maintain the integrity of the PCM and to drive centrosome separation during the early embryonic divisions.Citation12,31 Moreover, in the absence of Dplp the centrioles lose their orthogonal arrangement and start to partially fragment suggesting that Dplp may help the maintenance of centriole integrity. Remarkably, Dplp is localized to the basal body and not to the proximal centriole in Type I sensory neurons, consistent with a role for Dplp in basal body function.Citation44,45
The effect of Klp10A loss is not limited to centriole length as previously reported, but it also results in an abnormal distribution of Plk4, Sas-4 and Dplp.Citation28,29 We find, indeed, that these proteins spread along the length of the centrioles whereas they are restricted in control testes to the proximal region. An extended localization of the proximal centriole-associated proteins including Sas-4 and Dplp has been recently described in male germ stem cells of Asterless (Asl) mutants that also display overly long centrioles.Citation46 The finding of a similar phenotype in such different mutants point to the presence of a common target checking the localization of the basal proteins and monitoring centriole architecture and polarity.
How can the loss of Klp10A influence the localization of the basal proteins? A correlation between pericentrin and the minus end directed motor protein cytoplasmic dynein has been reported in human cultured cells and in Xenopus embryos where microtubule depolymerisation or dynein inactivation lead to failed pericentrin accumulation at the centrosome.Citation47,48 However, the observation that microtubules are dispensable for the localization of Dplp to the centrosome in early Drosophila embryos, points to alternative mechanisms in flies.Citation49 It has been suggested that the organization of internal centriole proteins may be involved in the proper positioning of the PCM components in the long Asl mutant male GSC centrioles.Citation46 Thus, mutations affecting the centriole architecture may result in the abnormal distribution of basal centriole-associated proteins.Citation46
The meiotic centrioles in Drosophila have a distinct proximal domain that keeps the overall organization seen in the short mitotic centrioles found in stem cells and spermatogonia. The main proteins involved in centriole assembly and centrosome organization are restricted to this region that closely matches the position of the cartwheel. The mid and distal regions of the centrioles are devoid of basal proteins and look like simple extensions of the centriole wall rather than true centrioles. Thus, the centriole functions appear to be essentially achieved by the basal domain.
A comparative analysis of germline stem cells, spermatogonia and primary spermatocytes, revealed that the cartwheel is disorganized in most of the mutant centrioles examined. Thus, the cartwheel or cartwheel associated components might play some roles to restrict the distribution of the basal proteins to the proximal region of the centriole. Sak/Plk4, Dplp and Sas-4 were, indeed, localized on the whole centriole fragments that lack cartwheel components. A possible hypothesis could be that the excessive elongation of the centrioles may depend by the fragmentation of the cartwheel leading to the loss of the basal properties.
However, both Asl and Klp10A elongated centrioles showed a distinct Sas-6 signal pointing to a remnant cartwheel.Citation28,46 Nevertheless, from conventional immunofluorecence analysis it is not possible to appreciate the true cartwheel structure.
The position of the daughter centrioles respect to the mother ones was unusual: in control testes the procentrioles always formed at the proximal end of the mothers, whereas in mutant testes the daughter centrioles had an ectopic position. This variation might be explained with the abnormal distribution of Plk4, the master protein involved in the assembly of the procentrioles and in the sequential recruitment of some centriole-associated proteins.Citation24,50-55
The broad distribution of Plk4 in mutant testes may enable more sites to recruit centriole-assembly proteins. Accordingly, the procentrioles in cep97 mutants were not always placed at the proximal end of the overly long mothers that also showed an extended localization of the basal proteins.Citation46 Therefore, Klp10A might be also involved in earlier stages of centriole duplication. Accordingly, the main defects in centriole organization were seen during the spermatogonial mitosis and in young primary spermatocytes when centrioles duplicate and start to elongate.
Sas-4 localization was more extended than Spd2 and Dplp in centrioles from 16-cell cysts of the spermatogonia and young primary spermatocytes in Klp10A testes, but as prophase progressed its distribution significantly reduces. By contrast, the localization of Dplp and Spd2 does not decrease. Therefore, Sas-4 is not required to maintain Dplp and Spd2 at the centriole. Fly centrioles, indeed, can still organize the PCM in the absence of Sas4.Citation56,57 This is surprising since Sas-4 would provide a scaffold for Dplp tethering at the mitotic centrosome.Citation58 The Cdk1-dependent phosphorylation of Sas-4 allows, indeed, the mitotic centrioles to duplicate and assemble functional centrosomes by promoting the incorporation of Asterless (Asl) that in turn plays a main role in recruiting Spd-2 during centrosome maturation.Citation3,4,51,34,59 Our observations suggest, therefore, that Sas-4 might play different roles in PCM organization during mitosis and meiosis and that its function is differently required during earlier and later stages of the male gametogenesis.
Materials and methods
Drosophila strains
OregonR flies were used as wild type. The Klp10A mutant line was previously described.Citation60 Observations were also made on a strain in which the male fertility was rescued by mobilization of the P-element with a P-element transposase.Citation28 In these flies we never observed abnormal localization of centriole associated proteins. Flies were raised on standard Drosophila medium at 24°C.
Antibodies
The following antibodies were used: chicken anti-Dplp (1:800,Citation61); rabbit anti-Spd2 (1:500,Citation40); mouse anti-Sas-4 (1:50,Citation58); rabbit anti Sak/Plk4 (1:50,Citation61); mouse anti-acetylated-tubulin (1:400; Sigma-Aldrich). Secondary antibodies used were Alexa Fluor 488 and 594 (1:800; Invitrogen).
Immunofluorescence preparations
Testes from mid aged pupae were dissected in phosphate buffered saline (PBS) and placed in a small drop of 5% glycerol in PBS on a glass slide. Testes were squashed under a cover glass and frozen in liquid nitrogen. The samples were then immersed in methanol for 10 min at −20°C. For antigen localization the samples were washed 15 minutes in PBS and incubated for 1 hour in PBS containing 0.1% bovine serum albumin (PBS-BSA) to block non specific staining. The samples were then incubated overnight at 4°C with the specific antisera in a humid chamber. After washing in PBS-BSA the samples were incubated for one hour at room temperature with the appropriate secondary antibodies. DNA was visualized following incubation of 3–4 minutes in 1 μg/ml Hoechst 33258 (Sigma). Samples were mounted in small drops of 90% glycerol in PBS.
Image acquisition
Images were taken by using an Axio Imager Z1 (Carl Zeiss) microscope equipped with an HBO 50W mercury lamp for epifluorescence and with an AxioCam HR cooled charge-coupled camera (Carl Zeiss). Gray-scale digital images were collected separately and pseudocolored and merged using Adobe Photoshop 7.0 software (Adobe Systems).
Transmission electron microscopy
Testes were isolated from mid-aged pupae and transferred in 2.5% glutaraldehyde buffered in PBS overnight at 4°C. Samples were subsequently rinsed in PBS and post-fixed in 1% osmium tetroxide in PBS for 2 hour at 4°C. The material was carefully washed in PBS, dehydrated in a graded series of Ethanol, embedded in a mixture of Epon-Araldite resin, and then polymerized at 60°C for 48 hr. Thin sections (60–70 nm thick) were obtained with a Reichert Ultracut E ultramicrotome equipped with a diamond knife, mounted upon copper grids, and stained with samarium triacetate and lead citrate. Samples were observed with a Tecnai Spirit Transmission Electron Microscope (FEI) operating at 100 kV and equipped with a Morada CCD camera (Olympus).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank A. Rodrigues-Martins and J. Gopalakrishnan for generously providing the antibodies.
Funding
This work was supported by a grant from MIUR (Grant 2012N7TX98 to GC).
References
- Bettencourt Dias M, Glover DM. Centrosome biogenesis and function: Centrosomics brings new understanding. Nat Rev Mol Cell Biol 2007; 8:451-63; PMID:17505520; http://dx.doi.org/10.1038/nrm2180
- Winey M, O'Toole E. Centriole structure. Philos Trans R Soc Lond B Biol Sci 2014; 369:20130457-20130457; PMID:25047611; http://dx.doi.org/10.1098/rstb.2013.0457
- Conduit PT, Richens JH, Wainman A, Holder J, Vicente CC, Pratt MB, Dix CI, Novak ZA, Dobbie IM, Schermelleh L, Raff JW. A molecular mechanism of mitotic centrosome assembly in Drosophila. eLife 2014; 3:e03399; PMID:25149451; http://dx.doi.org/10.7554/eLife.03399
- Conduit PT, Wainman A, Raff JW. Centrosome function and assembly in animal cells. Nat Rev Mol Cell Biol 2015; 16:611-24; PMID:26373263; http://dx.doi.org/10.1038/nrm4062
- Fu J, Lipinszki Z, Rangone H, Min M, Mykura C, Chao-Chu J, Schneider S, Dzhindzhev NS, Gottardo M, Riparbelli MG, et al. Conserved molecular interactions in centriole-to-centrosome conversion. Nat Cell Biol 2016; 18:87-99; PMID:26595382; http://dx.doi.org/10.1038/ncb3274
- Conduit PT, Wainman A, Raff JW. Centrosome function and assembly in animal cells. Nat Rev Mol Cell Biol 2015; 16:611-624; PMID:26373263; http://dx.doi.org/10.1038/nrm4062
- Jana SC, Marteil G. Bettencourt-Dias M.mMapping molecules to structure: unveiling secrets of centriole and cilia assembly with near-atomic resolution. Curr Opin Cell Biol 2014; 26:96-106; PMID:24529251; http://dx.doi.org/10.1016/j.ceb.2013.12.001
- Izquierdo D, Wang WJ, Uryu K, Tsou MF. Stabilization of cartwheel-less centrioles for duplication requires CEP295-mediated centriole-to-centrosome conversion. Cell Rep 2014; 8:957-65; PMID:25131205; http://dx.doi.org/10.1016/j.celrep.2014.07.022
- Wang WJ, Soni RK, Uryu K, Bryan Tsou MF. The conversion of centrioles to centrosomes: essential coupling of duplication with segregation. J Cell Biol 2011; 193:727-739; PMID:21576395; http://dx.doi.org/10.1083/jcb.201101109
- Fu J, Glover DM. Structured illumination of the interface between centriole and peri-centriolar material. Open Biol 2012; 2:120104; PMID:22977736; http://dx.doi.org/10.1098/rsob.120104
- Lawo S, Hasegan M, Gupta GD, Pelletier L. Subdiffraction imaging of centrosomes reveals higher-order organizational features of pericentriolar material. Nat Cell Biol 2012; 14:1148-58; PMID:23086237; http://dx.doi.org/10.1038/ncb2591
- Mennella V, Keszthelyi B, McDonald KL, Chhun B, Kan F, Rogers GC, Huang B, Agard DA. Subdiffraction-resolution fluorescence microscopy reveals a domain of the centrosome critical for pericentriolar material organization. Nat Cell Biol 2012; 14:1159-68; PMID:23086239; http://dx.doi.org/10.1038/ncb2597
- Sonnen KF, Schermelleh L, Leonhardt H, Nigg EA. 3D-structured illumination microscopy provides novel insight into architecture of human centrosomes. Biol Open 2012; 1:965-76; PMID:23213374; http://dx.doi.org/10.1242/bio.20122337
- Rodrigues–Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt–Dias M. From centriole biogenesis to cellular function: centrioles are essential for cell division at critical developmental stages. Cell Cycle 2008; 7:11-6; PMID:18196975; http://dx.doi.org/10.4161/cc.7.1.5226
- Gottardo M, Callaini G, Riparbelli MG. The Drosophila centriole - conversion of doublets into triplets within the stem cell niche. J Cell Sci 2015; 128:2437-42; PMID:26092937; http://dx.doi.org/10.1242/jcs.172627
- Hirono M. Cartwheel assembly. Phil Trans R Soc B 2014; 369:20130458; PMID:25047612
- Tates AD. Cytodifferentiation during spermatogenesis in Drosophila melanogaster: an electron microscopy study. PhD thesis, 1971, Rijksunivrsiteit de Leiden, The Netherlands.
- Fritz-Niggli H. Meiosis and spermatid formation in non-irradiated and irradiated male germ cells in Drosophila melanogaster. Rev Suisse Zool 1972; Suppl:245-65; PMID:4625128
- Riparbelli MG, Callaini G, Megraw TL. Assembly and persistence of primary cilia in dividing Drosophila spermatocytes. Dev Cell 2012; 23:425-32; PMID:22898783
- Gottardo M, Callaini G, Riparbelli MG. Aurora A inhibition by MNL8054 promotes centriole elongation during Drosophila male meiosis. Cell Cycle 2015; 14:2844-52; PMID:25785740
- Kim S, Tsiokas L. Cilia and cell cycle re-entry: more than a coincidence. Cell Cycle 2011; 10:2683-90; PMID:21814045
- Kobayashi T, Dynlacht BD. Regulating the transition from centriole to basal body. J Cell Biol 2011; 193:435-44; PMID:21536747
- Gottardo M, Callaini G, Riparbelli MG. The cilium-like region of the Drosophila spermatocyte: an emerging flagellum? J Cell Sci 2013; 126:5441-52; PMID:24105264
- Fu J, Hagan IM, Glover DM. The centrosome and its duplication cycle. Cold Spring Harb Perspect Biol 2015; 7:a015800; PMID:25646378
- Rogers GC, Rogers SL, Schwimmer TA, Ems-McClung SC, Walczak CE, Vale RD, Scholey JM, Sharp DJ. Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature 2004; 427:364-70; PMID:14681690
- Goshima G, Vale RD. Cell cycle-dependent dynamics and regulation of mitotic kinesins in Drosophila S2 cells. Mol Biol Cell 2005; 16:3896-907; PMID:15958489
- Mennella V, Rogers GC, Rogers SL, Buster DW, Vale RD, Sharp DJ. Functionally distinct kinesin-13 family members cooperate to regulate microtubule dynamics during interphase. Nat Cell Biol 2005; 7:235-45; PMID:15723056
- Delgehyr N, Rangone H, Fu J, Mao G, Tom B, Riparbelli MG, Callaini G, Glover DM. Klp10A, a microtubule-depolymerizing kinesin-13, cooperates with CP110 to control Drosophila centriole length. Curr Biol 2012; 22:502-9; PMID:22365849
- Franz A, Roque H, Saurya S, Dobbelaere J, Raff JW. CP110 exhibits novel regulatory activities during centriole assembly in Drosophila. J Cell Biol 2013; 203:785-99; PMID:24297749
- Woodruff JB, Wueseke O, Hyman AA. Pericentriolar material structure and dynamics. Philos Trans R Soc Lond B Biol Sci 2014; 369:20130459; PMID:25047613
- Lerit DA, Jordan HA, Poulton JS, Fagerstrom CJ, Galletta BJ, Peifer M, Rusan NM. Interphase centrosome organization by the PLP-Cnn scaffold is required for centrosome function. J Cell Biol 2015; 210:79-97; PMID:26150390
- Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol 2005; 15:2199-207; PMID:16326102; http://dx.doi.org/10.1016/j.cub.2005.11.042
- Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov AL, Raff JW. Flies without centrioles. Cell 2006; 125:1375-86; PMID:16814722; http://dx.doi.org/10.1016/j.cell.2006.05.025
- Novak ZA, Wainman A, Gartenmann L, Raff JW. Cdk1 phosphorylates Drosophila Sas-4 to recruit Polo to daughter centrioles and convert them to centrosomes. Dev Cell 2016; 37:545-57; PMID:27326932; http://dx.doi.org/10.1016/j.devcel.2016.05.022
- Riparbelli MG, Gottardo M, Glover DM, Callaini G. Inhibition of Polo kinase by BI2536 affects centriole separation during Drosophila male meiosis. Cell Cycle 2014; 13:2064-72; PMID:24802643; http://dx.doi.org/10.4161/cc.29083
- Kohlmaier G, Loncarek J, Meng X, McEwen BF, Mogensen MM, Spektor A, Dynlacht BD, Khodjakov A, Gonczy P. Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr Biol 2009; 19:1012-8; PMID:19481460; http://dx.doi.org/10.1016/j.cub.2009.05.018
- Schmidt TI, Kleylein-Sohn J, Westendorf J, Le Clech M, Lavoie SB, Stierhof YD, Nigg EA. Control of centriole length by CPAP and CP110. Curr Biol 2009; 19:1005-11; PMID:19481458; http://dx.doi.org/10.1016/j.cub.2009.05.016
- Tang CJ, Fu RH, Wu KS, Hsu WB, Tang TK. CPAP is a cell-cycle regulated protein that controls centriole length. Nat Cell Biol 2009; 11:825-31; PMID:19503075; http://dx.doi.org/10.1038/ncb1889
- Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. Plk4-induced centriole biogenesis in human cells. Dev Cell 2007; 13:190-202; PMID:17681131; http://dx.doi.org/10.1016/j.devcel.2007.07.002
- Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt-Dias M. Revisiting the role of the mother centriole in centriole biogenesis. Science 2007; 316:1046-50; PMID:17463247; http://dx.doi.org/10.1126/science.1142950
- Bornens M, Gönczy P. Centrosomes back in the limelight. Phil Trans R Soc B Biol Sci 2014; 369:20130452; http://dx.doi.org/10.1098/rstb.2013.0452
- Nigg EA, Stearns TT. The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol 2011; 13:1154-60; PMID:21968988; http://dx.doi.org/10.1038/ncb2345
- Callaini G, Whitfield WG, Riparbelli MG. Centriole and centrosome dynamics during the embryonic cell cycles that follow the formation of the cellular blastoderm in Drosophila. Exp Cell Res 1997; 234:183-90; PMID:9223385; http://dx.doi.org/10.1006/excr.1997.3618
- Martinez-Campos M, Basto R, Baker J, Kernan M, Raff JW. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J. Cell Biol 2004; 165:673-83; PMID:15184400; http://dx.doi.org/10.1083/jcb.200402130
- Galletta BJ, Guillen RX, Fagerstrom CJ, Brownlee CW, Lerit DA, Megraw TL, Rogers GC, Rusan NM. Drosophila pericentrin requires interaction with calmodulin for its function at centrosomes and neuronal basal bodies but not at sperm basal bodies. Mol Biol Cell 2014; 25:2682-94; PMID:25031429; http://dx.doi.org/10.1091/mbc.E13-10-0617
- Galletta BJ, Jacobs KC, Fagerstrom CJ, Rusan NM. Asterless is required for centriole length control and sperm development. J Cell Biol 2016; 213:435-50; PMID:27185836; http://dx.doi.org/10.1083/jcb.201501120
- Purohit A, Tynan SH, Vallee R, Doxsey SJ. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J Cell Biol 1999; 147:481-92; PMID:10545494; http://dx.doi.org/10.1083/jcb.147.3.481
- Young A, Dictenberg JB, Purohit A, Tuft R, Doxsey SJ. Cytoplasmic dynein-mediated assembly of pericentrin and gamma tubulin onto centrosomes. Mol Biol Cell 2000; 11:2047-56; PMID:10848628; http://dx.doi.org/10.1091/mbc.11.6.2047
- Kawaguchi S-I, Zheng Y. Characterization of a Drosophila centrosome protein CP309 that shares homology with Kendrin and CG-NAP. Mol Biol Cell 2004; 15:37-45; PMID:14565985; http://dx.doi.org/10.1091/mbc.E03-03-0191
- Cizmecioglu O, Arnold M, Bahtz R, Settele F, Ehret L, Haselmann-Weiss U, Antony C, Hoffmann I. Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome. J Cell Biol 2010; 191:731-9; PMID:21059844; http://dx.doi.org/10.1083/jcb.201007107
- Dzhindzhev NS, Yu QD, Weiskopf K, Tzolovsky G, Cunha-Ferreira I, Riparbelli M, Rodrigues-Martins A, Bettencourt-Dias M, Callaini G, Glover DM. Asterless is a scaffold for the onset of centriole assembly. Nature 2010; 467:714-8; PMID:20852615; http://dx.doi.org/10.1038/nature09445
- Hatch EM, Kulukian A, Holland AJ, Cleveland DW, Stearns T. Cep152 interacts with Plk4 and is required for centriole duplication. J Cell Biol 2010; 191:721-9; PMID:21059850; http://dx.doi.org/10.1083/jcb.201006049
- Kim TS, Park JE, Shukla A, Choi S, Murugan RN, Lee JH, Ahn M, Rhee K, Bang JK, Kim BY. et al. Hierarchical recruitment of Plk4 and regulation of centriole biogenesis by two centrosomal scaffolds, Cep192 and Cep152. Proc Natl Acad Sci USA 2013; 110:E4849-57; PMID:24277814; http://dx.doi.org/10.1073/pnas.1319656110
- Sonnen KF, Gabryjonczyk AM, Anselm E, Stierhof YD, Nigg EA. Human Cep192 and Cep152 cooperate in Plk4 recruitment and centriole duplication. J Cell Sci 2013; 126:3223-33; PMID:23641073; http://dx.doi.org/10.1242/jcs.129502
- Klebba JE, Galletta BJ, Nye J, Plevock KM, Buster DW, Hollingsworth NA, Slep KC, Rusan NM, Rogers GC. Two polo-like kinase 4 binding domains in asterless perform distinct roles in regulating kinase stability. J Cell Biol 2015; 208:401-14; PMID:25688134; http://dx.doi.org/10.1083/jcb.201410105
- Stevens NR, Raposo AA, Basto R, St Johnston D, Raff JW. From stem cell to embryo without centrioles. Curr Biol 2007; 17:1498-503; PMID:17716897; http://dx.doi.org/10.1016/j.cub.2007.07.060
- Riparbelli MG, Callaini G. Male gametogenesis without centrioles. Dev Biol 2011; 349:427-43; PMID:20974123; http://dx.doi.org/10.1016/j.ydbio.2010.10.021
- Gopalakrishnan J, Mennella V, Blachon S, Zhai B, Smith AH, Megraw TL, Nicastro D, Gygi SP, Agard DA, Avidor-Reiss T. Sas-4 provides a scaffold for cytoplasmic complexes and tethers them in a centrosome. Nat Commun 2011; 2:359; PMID:21694707; http://dx.doi.org/10.1038/ncomms1367
- Novak ZA, Conduit PT, Wainman A, Raff JW. Asterless licenses daughter centrioles to duplicate for the first time in Drosophila embryos. Curr Biol 2014; 24:1276-82; PMID:24835456; http://dx.doi.org/10.1016/j.cub.2014.04.023
- Peter A, Schottler P, Werner M, Beinert N, Dowe G, Burkert P, Mourkioti F, Dentzer L, He Y, Deak P, et al. Mapping and identification of essential gene functions on the X chromosome of Drosophila. EMBO Rep 2002; 3:34-8; PMID:11751581; http://dx.doi.org/10.1093/embo-reports/kvf012
- Rodrigues-Martins A, Bettencourt-Dias M, Riparbelli M, Ferreira C, Ferreira I, Callaini G, Glover DM. DSAS-6 organizes a tube-like centriole precursor, and its absence suggests modularity in centriole assembly. Curr Biol 2007; 17:1465-72; PMID:17689959; http://dx.doi.org/10.1016/j.cub.2007.07.034
