Experimental evidence in the unicellular choanoflagellate Salpingoeca rosetta, the closest living relative of animals, proposes that emergence of multicellularity – one of the founding step of animal life – is caused by incomplete separation of daughter cells at the last step of cell division, cytokinesis.Citation1 Incomplete cytokinesis also occurs in animals, giving rise to polyploid cells (hepatocytes) or syncitia (germ cells). Until recently cytokinesis was viewed as a mechanism orchestrated only by cell intrinsic factors,Citation2 yet in Salpingoeca Rosetta the switch from unicellular to multicellular state can be driven by extrinsic factors such as predators, changes in ocean chemistry or emergence of new ecological niches.Citation1 These observations indicate that completion of cytokinesis may be controlled by factors present in the environment of dividing cells.
In our recent study,Citation3 we asked whether cytokinesis is similarly controlled by extracellular cues in mammals, focusing on the role of local communication via Eph/ephrin signaling. Eph receptor tyrosine kinases and their membrane-bound ligands, ephrins, are best known for their role in modulating cell adhesion and migration, mostly through the regulation of the actin cytoskeleton.Citation4 Using live and fixed cell microscopy, we observed in various cell lines that activation of the EphB2 receptor induced cytokinesis delays and/or failures, which correlated with an increased number of polyploid cells. We showed that EphB2 tyrosine kinase activity was necessary for cytokinesis defects and we identified Citron kinase (CitK) as a downstream target of EphB2 signaling. CitK is a serine/threonine kinase that plays a well characterized function in cytokinesis.Citation5 We found that CitK interacts with EphB2; that it is phosphorylated on tyrosine upon activation of Eph signaling and that this phosphorylation is mediated by Src. Using Mass Spectrometry we identified 2 tyrosine residues located in the Rho Binding domain of CitK that are phosphorylated by Src. Functional assays with engineered phosphomimetic and unphosphorylatable mutants of CitK confirmed that phosphorylation of these residues modulates CitK interaction with RhoA and alters cytokinesis.
To demonstrate the physiological relevance of these in vitro observations, we focused on dividing neural progenitors (NPs) of the neocortex since Eph receptors and ephrins are expressed in these cells and CitK has been shown in mouse, rat and humans to play a critical role in NP cytokinesis.Citation6 We showed that CitK and EphB2 partially co-localize in dividing NPs in vivo and that CitK is phosphorylated on tyrosine in these cells. More importantly, we found that pups lacking EphB2 signaling exhibit a decrease in the fraction of polyploid cortical neurons which we used as a read-out of NP cytokinesis failure.Citation3
Thus, our study reports for the first time a complete molecular cascade downstream of Eph signaling controling cytokinesis. Interestingly, all the actors of this cascade are present in the genome of Salpingoeca rosetta: Eph receptors, Src kinase and a citron kinase-like protein.Citation1,7 In addition, our study confirms that cytokinesis can be controlled by cues present in the environment of dividing cells in mammals and suggests that CitK plays a key role in the completion of cytokinesis as an integrator of both intrinsic and extrinsic information.
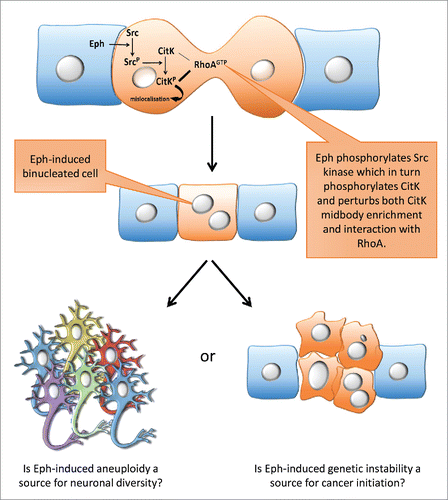
Our findings raise a number of questions (), first with respect to the role of Eph/ephrin signaling in cancer. Indeed, Eph signaling deregulation is observed in many cancers, but until now its role was restricted to metastatic diffusion and tumor aggressiveness. Our data showing that Eph-induced cytokinesis failure leads to aneuploidy, suggests that Eph/ephrin signaling may play a role in cancer initiation by promoting genetic instability.
More puzzling questions raised by our study concern the observation that Eph/ephrin signaling controls neuronal polyploidy. In mammals, the majority of cells are diploid except for a handful of cell types (gametes, hepatocytes and cardiomyocytes). The existence of polyploid neurons in mammals was reported several years ago, yet little is known about these neurons. Is there an advantage for neurons in being polyploid? Is polyploidy an additional source of neuronal diversity and does it participate in the complexity of the mammalian brain? What is the significance of its regulation by local signaling via Eph/ephrin?
In conclusion, modulation of cytokinesis by environmental information appears as an important mechanism driving evolution, from emergence of multicellularity to generation of neuronal diversity.
Figure 1. An Eph-induced signaling cascade prevents cytokinesis completion: possible physiological and pathological consequences. Activation of Eph signaling in dividing cells interrupts or delays cytokinesis which increases the fraction of polyploid cells. In the neocortex polyploid neurons could participate in generating neuronal diversity while in other tissues, cytokinesis defects could increase genomic instability and participate in tumor initiation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Richter DJ, King N. The genomic and cellular foundations of animal origins. Annu Rev Genet 2013; 47:509-37; PMID:24050174; http://dx.doi.org/10.1146/annurev-genet-111212-133456
- Fededa JP, Gerlich DW. Molecular control of animal cell cytokinesis. Nat Cell Biol 2012; 14:440-7; PMID:22552143; http://dx.doi.org/10.1038/ncb2482
- Jungas T, Perchey RT, Fawal M, Callot C, Froment C, Burlet-Schiltz O, Besson A, Davy A. Eph-mediated tyrosine phosphorylation of citron kinase controls abscission. J Cell Biol 2016; 214:555-69; PMID:27551053; http://dx.doi.org/10.1083/jcb.201602057
- Kania A, Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol 2016; 17:240-56; PMID:26790531; http://dx.doi.org/10.1038/nrm.2015.16
- Gai M, Camera P, Dema A, Bianchi F, Berto G, Scarpa E, Germena G, Di Cunto F. Citron kinase controls abscission through RhoA and anillin. Mol Biol Cell 2011; 22:3768-78; PMID: 21849473; http://dx.doi.org/10.1091/mbc.E10-12-0952
- Di Cunto F, Imarisio S, Hirsch E, Broccoli V, Bulfone A, Migheli A, Atzori C, Turco E, Triolo R, Dotto GP, et al. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron 2000; 28:115-27; PMID:11086988; http://dx.doi.org/10.1016/S0896-6273(00)00090-8
- King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, Fairclough S, Hellsten U, Isogai Y, Letunic Is, et al. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 2008; 451:783-8; PMID:18273011; http://dx.doi.org/10.1038/nature06617
